- Login
- Home
- About the Initiative
-
Curricular Resources
- Topical Index of Curriculum Units
- View Topical Index of Curriculum Units
- Search Curricular Resources
- View Volumes of Curriculum Units from National Seminars
- Find Curriculum Units Written in Seminars Led by Yale Faculty
- Find Curriculum Units Written by Teachers in National Seminars
- Browse Curriculum Units Developed in Teachers Institutes
- On Common Ground
- Publications
- League of Institutes
- Video Programs
- Contact
Have a suggestion to improve this page?
To leave a general comment about our Web site, please click here
HIV: From Horror to Hope
byTimothy Kennedy SpenceIntroduction and Rationale
When I was little, I was obsessed with the vastness of the night sky. To me, it was a dark sea of endless possibilities. I embraced a culture steeped in the thrill of science fiction, enthralled by the glamour of the early astronauts. My bedroom walls were full of artistic renderings of blighted extraterrestrial landscapes, and my bookcase was crammed with anything that had to do with outer space. I tell you these things to make it clear that I was a very motivated learner as a child, and that similar motivation happens to all kinds of people all of the time. It also doesn't happen to other people so readily. As a high school teacher who teaches various levels of math, I see both kinds of people every day.
Lack of student motivation is not a new problem, but I have noticed an uptick in it recently. I have been teaching math for ten years, and I am now very aware that teaching math in a lecture format to a bunch of sitting teenagers can be, for some, no more than a dull chore that has little to do with their technologically advanced, fast paced world. Those technological developments have changed our learning environment, and the time is now to consider alternative ways of presenting math. With that in mind, I have found that making connections between math and science has been the best way for me to tap into my student's natural curiosity and passion, and that is what I have strived for from the start.
My degree is in Chemistry, so my math courses have been peppered with dashes of chemistry and other science applications. I teach Algebra II and Calculus among other courses, so I really do have a pool of motivated, highly intelligent, and sure to be successful students who I work with. Many who make it to my Calculus class are headed to the sciences or engineering. I may have had my eyes on the skies when I was their age, but times have changed, and their focus is a little more earthbound. More and more of my students are considering a career in biotech and the biological sciences.
I have chosen the study of the most famous retrovirus, HIV-1, as the topic that I want to integrate into my Algebra II curriculum for several reasons. Human gene therapy may have experienced setbacks in past years, but it is certain to continue to play a major role in medical research. Retroviral vectors are probably the most widely used delivery vehicles in gene therapy because of their efficiency and precision of integration. 1 HIV-1 has some additional characteristics which can make it an even better candidate for some applications.
For a student considering a career in biology or medicine, this could be a very interesting and perhaps inspirational topic that he or she may have little or no knowledge of. Biology is taught at our school, but the curriculum is limited, and many of the more advanced students bypass it in favor of Chemistry. In any event, I like the idea of elevating my student's knowledge beyond the standards.
I would also like to explore HIV-1, which has played a major role in my life and the lives of many people I have known. Most sixteen year olds know some things about HIV, but probably not so much about the AIDS epidemic, and probably less about the ongoing battle to find a vaccine and a cure, and the new ways that HIV is being used in genetic engineering. There has never been a more fascinating disease, in terms of its mechanism, its impact on medical research and the pharmaceutical profession, and its political consequences. Although my class is a math class, I want to take some time to explore this section. I think it could be a good way to get things started, to create a sort of 'viral scaffolding'.
Algebra II contains a unit that deals with probability, and this will constitute the math part of the lesson. I have wanted to find an extracurricular topic to use as a real world application that the students could benefit from, and this should be a very good vehicle for presenting probability in a fresh and unique way. I plan to focus on topics involving HIV testing, viral mutation, and drug resistance as a basis for teaching probability.
My unit will be inquiry based, as I won't be sure just how much the students do know, and my hope is that they will have questions that I hadn't even considered. I encourage discussion. I plan on using pamphlets, handouts, and animations on my Smartboard as a delivery method for the information. There is no time for lengthy reading, and the animations that I have seen on the Web make some of the more complicated biological processes much easier to understand. They are perfect for students who have trouble with reading comprehension, and there are plenty of them, even in Algebra II. There will be an assessment on the unit, which in Algebra II, means some sort of test.
Finally, Algebra II seems to me a good point in a student's career to be exposed to something that integrates math, science, and the real world, because some of them are already thinking about their futures, and they still have a few years to do that. My hope is to spark some interest in a very relevant topic by stepping outside of my everyday math curriculum and see what happens. My students are probably not so different than I was when I was their age, looking out at their own dark sea of endless possibilities, and wondering what is coming next. I would like to provide a spark that, for at least some of them, might shed a little light on their path.
The Early History of HIV/ AIDS
Research in recent years has strengthened the theory that a virus that infects African monkeys, which is now known as SIV (Simian Immunodeficiency Virus), jumped from these monkeys to humans sometime during the 1930s. 2 This is thought to have happened when blood from an infected chimpanzee was transferred to the blood of a human during the slaughter of the animal for consumption. Between those years and 1980, there were a small number of unexplained deaths accompanied by wasting and rarely seen infections, first in Africa, and later among several Haitians and other foreigners who had traveled extensively in the Congo and other African countries.
The first case to be officially recognized by the Center for Disease Control (CDC) in the United States came on April 24, 1980. The man was a San Franciscan by the name of Ken Home, who presented with Kaposi's Sarcoma, a rare form of skin cancer seen only occasionally in elderly men of Mediterranean descent. 3 Later that same year, a French Canadian flight attendant named Gaetan Dugas, who traveled regularly to Africa, began to frequent the gay bathhouses of New York City. He would later receive the dubious honor of being named "Patient Zero", after his name was connected via way of sexual contact to some of the earliest AIDS cases in the United States. 4
The New York Times first reported the disease in 1981, with the first death in New York City coming early in the year. 5 By that summer, the CDC was reporting outbreaks of Kaposi's Sarcoma, and Pneumocystis Carinii pneumonia (PCP), a rare form of pneumonia usually found only in severely immunosuppressed patients, among gay men in New York City, San Francisco, and Los Angeles. 6 Because of this, the virus came to be known as GRID (gay-related immune deficiency). 7 These infections were so aggressive that by the end of that year, nearly one hundred people had died.
In June of the following year, NBC aired the first national televised report on the new disease. In July, when the CDC identified a cluster of Haitian immigrants who had similar symptoms, the name of the virus was changed from GRID to AIDS (acquired immune deficiency syndrome), due largely to the efforts of gay community leaders, as it became clear that this disease was not strictly limited to homosexual men. By the end of the year, more than six hundred people had died.
During 1983, Luc Montagnier, a French virologist, isolated a new human retrovirus from a patient with AIDS symptoms at the Pasteur Institute in France. It was given the name LAV (lymphadenopathy-associated virus). 8 The following year, in San Francisco, Dr. Jay Levy also isolated a retrovirus from a group of patients there, comprised mostly of gay white males. He termed it ARV (AIDS-associated virus). 9
Finally, in 1984, an American biologist name Robert Gallo, a pioneer in the study of retroviruses, published several papers stating that AIDS was definitely caused by a new retrovirus, and changed the name once again to HTLV-3 (human T-lymphotropic virus type 3), closely relating it to the already discovered HTLV-1 and HTLV-2 retroviruses. Eventually, the virus came to be known as HIV-1. Although Luc Montagnier was awarded the Nobel Prize for his work, most experts credit both men with the discovery of this strange new retrovirus. 10 By the end of that year, the worldwide death toll had reached an alarming 5, 596 people.
Viruses
What is a virus, and how does it work?
A virus is a piece of genetic material (DNA or RNA) that is enclosed in a protective coat usually referred to as a capsid. It is the simplest forms of life, so simple, in fact, that there is some debate over whether it is a life form at all. It is believed that most viruses which infect humans evolved relatively recently, in the past five to ten thousand years, possibly as a consequence of our increasingly rapid evolution. 11 Viruses are basically parasites which have 'learned' to hijack a cell. Once inside, they use the cell's own machinery to reproduce, assemble, package, and protect the newly produced viruses, which can survive outside the cell, and thereby propagate infection to other cells.
There are now about fifty different viruses that cause disease in humans. 12 In contrast to bacteria, some of which are beneficial or even essential for our survival, a virus which contributes any positive health benefit at all has never been discovered. To have survived successfully, a virus needs a method of entry into its 'host' cell, and the ability to replicate once it gets there. It must also develop a way to thwart the host's natural defenses until it can establish an infection. Finally, the virus must figure out a means of transmission from one host to another; in short, to get out while the getting is good.
What happens when a virus enters a human host?
Humans have several types of defenses against viruses. The initial defense is the physical barriers we are born with; our skin, protective cells in our respiratory and reproductive tracts, and acidic conditions and destructive enzymes in our digestive tract. Because of the enormous number of viruses in existence, this defense is likely to be overwhelmed at some point.
The next defense in line is known as the innate defense system. It includes the phagocytes, which function as 'garbage eaters', and the neutrophils, a white blood cell whose job is to kill foreign agents that present in the blood. There are also about twenty different proteins that bind to the surface of any of these foreign objects, and tag them for destruction by one of these killer cells.
When the number of viruses overwhelms this system, the adaptive immune system comes into play. This involves several other types of white blood cells; B cells, killer T cells, T helper cells, and dendritic cells. B cells manufacture antibodies on demand for the purpose of identifying and tagging foreign invaders outside of the cell, much like the proteins mentioned above. The killer T cells, by contrast, have the ability to recognize what is happening inside of the cell, by scanning proteins on the cell surface. If these proteins exhibit properties of viral proteins, the killer T cell destroys the cell and the virus within.
Any one killer T cell has the ability to detect only one protein, but they are differentiated such that, together, their sum total can recognize any viral protein. However, the system needs time to manufacture enough of the particular killer T cells that are specific to the recognized viral protein, in the same way that the B cell manufactures antibodies. These processes can take up to a week or more. Unfortunately, this delay is what gives the virus time to establish itself in the host cell, and it is a critical factor for its survival.
Supervising both these operations of the adaptive immune system are the dendritic cells and the T helper cells, which might be thought of as messengers of this system, carrying information which orchestrates the entire process. Dendritic cells move to the lymph nodes, sometimes with the virus to be identified in tow, activating the T helper cells, which, in turn, assist in the activation of B cells and killer T cells. Some of these B and killer T cells will remain in the host as 'memory' cells, meaning they are always present, and ready to mount a quick defense against any subsequent viral infection that they 'remember'.
Finally, there is the interferon defense system. Human cells have sensor molecules which can detect a viral footprint, and turn on a signal which begins production of proteins called type 1 interferon. Interferon works within the cell to chemically halt viral reproduction, often killing the cell along with the virus inside it. It also moves out of the infected cell and warns other cells in the vicinity that they may be in danger of infection. Every known virus activates this defense system in the human body, and the most successful viruses have developed very clever ways to evade it. 13
Retroviruses
What is a Retrovirus?
The central dogma of biology states that genetic information in DNA is transcribed into messenger RNA (mRNA), which then codes for translation of a particular protein for the purpose of gene expression. The retroviruses are a family of viruses that do not abide by this convention. They were recognized over a hundred years ago as cancer causing agents in animals and named RNA viruses, but it was not until about 1970 that their mechanism began to be understood. 14 The discovery of an enzyme in the virus particle, now known as reverse transcriptase (RT), was determined to copy the single strand RNA genome of the virus into double stranded DNA, soon after its entry into the host cell. This sequence of events is the very opposite of that proposed by central dogma, and as their transcription appeared to proceed in reverse, they were given the name retrovirus.
The First Human Retrovirus, HTLV-1
In 1980, the first retrovirus that infects humans was discovered and named HTLV-1 (human T cell leukemia virus). 15 It is estimated that this virus has been a part of the genome of very specific human populations as far back as perhaps one hundred thousand years. Its mechanism of infection is typical of all retroviruses, but it also has some unique characteristics that set it apart from retroviruses found in animals. It targets T helper cells and dendritic cells.
HTLV-1 enters these cells with the help of certain proteins on the cell surface that assist in the binding of the virus envelope to the cell, and then in the fusion of the viral envelope and the membrane of the cell. When the fusion is complete, the RNA genome, which is composed of two identical single-stranded RNA molecules, is released into the cytoplasm. The capsid is removed, and the reverse transcriptase, which comes packaged with the virus, begins copying the RNA genome into a single strand of complementary DNA (cDNA), and then destroys the original RNA genome. The reverse transcriptase then makes a complementary copy of this single DNA strand, producing a double stranded cDNA molecule which contains all the genetic information of the virus. The cDNA molecule then moves into the nucleus of the cell, and with the help of another viral enzyme called integrase (IN), it integrates the genetic material directly into the DNA of the infected cell. Once integration occurs, the viral genome is referred to as a provirus.
The provirus is now capable of producing a lifelong infection in the host, because when the infected cell replicates, the HTLV-1 genome is passed to each of the daughter cells. There are several additional proteins packaged with HTLV-1, one of which is named Tax. It stimulates the cell to replicate, and therefore infected cells propagate more quickly than the non-infected cells, thus producing greater numbers of infected cells. The provirus is now capable of using the cells own RNA polymerase to either make short messenger RNAs (mRNAs) for the purpose of viral protein production, or the entire genome which will become new virus particles. Two of these RNA genomes eventually group together, and along with a set of both viral and cellular proteins, bud through the cell membrane, borrowing a protective envelope as they exit, and leave the cell to begin new infections in neighboring cells.
One limitation to the proliferation of HTLV-1 infection is that the integration of the provirus cannot occur unless the target cell is active, in other words, when it is in the process of replicating its DNA. Since the vast majority of these cells are at rest at any given time, most of them are not susceptible to infection. Also, when infection does occur, the number of viruses produced inside of a cell by HTLV-1 is relatively few, so their exit does not result in cell rupture and subsequent cell death. HTLV-1 therefore establishes what is often referred to as a latent infection. 16
On the other hand, this low level of activity also benefits the virus. Lower virus count means less viral RNA and viral proteins that can be detected by the host immune system, the killer T cells in particular, so the virus operates relatively undetected. Another advantage the virus has is that when it does proliferate, it is able to disguise several of its proteins to avoid detection when it is in the process of budding from the cell. It also has the ability to spread by direct cell to cell contact without budding at all. In general, because of its ability to hide its activity from the host immune system, it minimizes the response against it, and this a primary reason that this virus has survived successfully for so long.
Although HTLV-1 is not aggressively pathogenic, and most people are not even aware that they are infected, a small percentage of them will eventually experience enough T cell depletion that they become susceptible to development of several types of opportunistic infections. About two percent contract a disease called ATL (adult T cell leukemia), but this normally does not present until forty or more years after the initial infection. 17 It is believed that this virus, although it is closely related to the much more virulent HIV-1 virus that causes AIDS, has achieved a certain degree of equilibrium with its human host over the course of a hundred thousand years of co-evolution. 18
HIV
HIV-1 is a retrovirus.
As stated above, HIV-1 is closely related to the HTLV-1 and HTLV-2, as all are human retroviruses. In fact, at first, Dr. Robert Gallo named the virus HTLV-3, but it quickly became clear that this virus was different. 19 The virus was renamed HIV-1, and it was categorized as a lentivirus, the prefix 'lenti' meaning slow. 20 They are a subset of the retroviruses that have the unique ability to infect non-dividing cells.
Nonetheless, HIV-1 and HTLV share many characteristics and mechanisms. They both infect the same immune system cells, which are cells that have CD4 surface proteins, such as T helper cells and dendritic cells. The life cycle of HIV with in the cell, is very similar to HTLV, and is described in the previous section. When the virus is released, infection spreads directly to the surrounding cells. Infected dendritic cells often carry the virus directly into the lymph nodes where these T helper cells congregate, providing large numbers of target cells for HIV to infect. The virus is then carried throughout the body by the blood and the lymph, and a systemic infection is established.
Another similarity between the viruses is that they both establish a latent infection. Their genome is integrated into the host genome, and, at certain times, produce such a low level of cellular activity that they are unable to be detected by the host immune system. These 'inactive' cells have come to be referred to as virus reservoirs, and can be thought of as a viral 'safe space'. This latent infection can take several days to set up, but that is usually enough time before the host adaptive immune system is ready to mount its response.
HIV also has some profound distinctions. Unlike HTLV, HIV usually kills the cells it infects because it produces far greater numbers of virus, which tear up the cellular membrane upon mass exit. For HTLV infection to occur, the target cell must be active. This is not true of the more aggressive HIV virus, which can infect some cells that are not proliferating at all. This strengthens the latent infection, but HIV really prefers a proliferating cell which has the ability to manufacture large amounts of new virus. When this happens, the immune response is powerful, but HIV even uses this to its advantage. It hijacks the entire system, infecting the now rapidly proliferating T helper cells, and other immune system cells which are designed to fight it. As more T helper cells are produced for the purpose of fighting the infection, they are infected and destroyed by the HIV virus, releasing ever more virus and producing a rapid and often devastating systemic infection.
In the early years of the AIDS epidemic, when little about HIV was understood, and there was really no treatment of any kind available, death from AIDS due to HIV infection was often swift and terrifying. However, even then, in many patients infected with HIV, this initial infection was often followed by a chronic phase, during which the patient remained either asymptomatic or demonstrated only mild symptoms. This is typical of HTLV, but as HIV is a much more aggressive virus, this phase does not last so long. As time wears on, the total number of T cells decreases as production of new ones cannot keep pace with the destruction of infected ones. After perhaps several years, there are not enough T helper cells to assist the killer T cells, and the virus eventually overwhelms the body's natural defenses, leaving the patient vulnerable to a host of pathogens, which, under normal circumstances, would rarely present a problem.
It is estimated that, today, thirty years after those frightening years at the beginning of the AIDS epidemic, there may be as little as a fraction of one percent of patients from that time who are still alive.
Anti-Retroviral Drug Therapy for Treatment of AIDS
Fortunately, treatment of HIV using anti-retroviral drug therapy has made it the chronic but manageable disease that it has become today. Azidothymidine (AZT) was the first, and for some time, the only approved drug available, but today, the number and variety of medications used to treat HIV has increased dramatically. Many have been fast tracked or have received accelerated approval by the FDA, especially during those early years when time was of the essence. There have been many drug and vaccine trials, with varying degrees of success and failure. Countless alternative therapies have come and gone, often representing the final glimmer of hope for AIDS patients desperate to survive.
One other notable aspect of HIV-1 not mentioned in the previous section is its extreme genetic variability. This produces mutant strains not only from host to host, but also in strains within a single individual. All retroviral reverse transcriptase (RT) is highly error prone when compared to cellular DNA, which has an error frequency of about one mistake per billion nucleotides copied, but HIV RT has one of the highest error frequencies ever seen in any virus. 21 This rapid genetic change over time is thought to contribute to the prolonged and progressive nature of the infection by allowing the mutating virus to avoid recognition by the host immune system. These mutations also have implications for current day drug therapies, because these variant unrecognizable strains are likely to produce drug resistance and subsequent treatment failure. AZT was shown to be resistant in some patients as early as 1989. 22
Today, combination therapy, meaning multiple drugs, is used when treating HIV. This is particularly true for long term survivors, whose virus has likely undergone many mutations. Regardless of the variety of names these drugs are given, all of them basically inhibit some mechanism of the virus at some point in its life cycle, as detailed in an earlier section. Most of these drugs, therefore, are referred to as 'inhibitors'.
The first point at which the virus can be attacked is at the entry level, thus the class of drugs which do this are called Entry Inhibitors (EI). Maraviroc binds to and distorts CCR5, a protein on the T cell surface to which HIV binds. 23 Fuzeon, also known as T20, is an injectable fusion inhibitor that binds to gp41, and interferes with process of bringing the virus and cell into direct contact. 24 There are also other receptors the virus can use, so as can be seen, there are multiple ways in which a virus can enter a cell, and research continues to develop multiple ways to stop that from happening.
The next step at which the life cycle can be disrupted is the RNA to cDNA replication, and this class of drugs is referred to as Reverse Transcriptase Inhibitors (RTI). AZT is an early nucleoside RTI, and it works by posing as a nucleotide, and then interrupting the growing DNA chain, because its 3' carbon end is defective. 25 Truvada is a newer nucleoside RTI drug, and has less toxic side effects than AZT. There are also variations of RTIs available called nucleotide RTIs, such as Tenofovir. 26 Both of these are known as competitive substrate inhibitors because they substitute into the viral replication. Non-nucleoside RTIs, such as Intellence 27, use a completely different mechanism. They inhibit the movement of several proteins which are necessary to synthesize the cDNA molecule, and are thus called non-competitive inhibitors. Like the entry inhibitors, there are a variety of inhibitor options.
The integration of the viral genome into the host DNA can be prevented by using an Integrase Inhibitor. This class of drugs is relatively new, and Isentress, the first of its kind, was approved by the FDA in late 2007, after a very successful trial. 28 There are also variations in the way these drugs prevent the host DNA from being cleaved, facilitating the insertion of the viral genome.
During the final assembly stage of the new viral particles, an enzyme called HIV protease cuts the long polypeptide chain into shorter pieces that become the functional proteins that travel with the virus when it exits the cell. Because there is no equivalent enzyme in the cell that the virus could use instead, this is an ideal point at which to interrupt the virus. There are now many Protease Inhibitors on the market, and they have become increasingly effective since their introduction on December 6, 1995. The first was named Saquinavir, and was released through the FDA's accelerated approval program, marking the turning point in the war on AIDS. 29
HIV and Gene Therapy
The purpose of gene therapy is to modify the genetic material of living cells to produce a positive health outcome. It involves the insertion of a functional gene that contains some kind of information sequence. There are two types of gene therapy, somatic cell and germ line. A procedure using the somatic cell method can potentially eliminate the effects of a disease, without passing the inserted gene to the patient's offspring. Germ line therapy passes the inserted gene, because the reproductive cells will carry the new gene.
Prior to 1996, scientists mainly used modified retroviruses when gene transfer into the chromosomes of target cells was needed, and adenovirus vectors when such integration was not needed. A vector is simply a name given to genetic material that carries information into a cell. However, there has been little success in gene transfer with such virus vectors because even though the vectors can enter into their target cells, the cells need to be dividing to break the nuclear membrane so the gene can enter and integrate into the chromosome.
Scientists have realized that because members of the lentivirus subfamily, to which HIV belongs, also have the ability to transfer genetic material into the genomes of non-dividing cells, they might be a good choice to use as a vector. As it turns out, lentiviruses do provide highly effective gene therapy, and can change the expression of a target cell's gene for up to six months. 30 Gene therapy using lentiviruses and HIV-1 has been the focus of a number of labs during the past few years.
HIV has a unique 'matrix' protein that contains a localization sequence which is recognized by the import machinery of the nucleus of many cells. Once inside the cytoplasm of the cell, the nuclear import machinery docks the complex at a nuclear membrane pore, and then lets it pass into the nucleus. Lentiviruses can be used with differentiated cells such as neurons, macrophages, hematopoietic stem cells, retinal photoreceptors, and muscle and liver cells, cell types for which previous gene therapy methods could not be used. 31
HIV contains a single stranded RNA-genome that is approximately 10 kb long, flanked with long terminal repeats (LTR) that aid in the initiation of the transcription of a particular gene. Being a very aggressive virus, it is also a strong promoter, which means that it is highly successful at being taken up by the target cells. It has been shown that the HIV vector has a higher rate of expression in target cells than any other retrovirus The vector is designed so that a genetic sequence is cloned into a region that is flanked by LTRs, promoting integration of that gene into the genome of the target cell, just as the LTRs in HIV integrate the cDNA into the host chromosome. Certain proteins are not included, and the virus particles produced are replication deficient, and are therefore unable to continue to infect their host after the therapeutic gene is delivered.
The vectors are produced ex vivo, meaning they are manufactured outside of the host. The cells carrying the vector are then reintroduced in the patient for the therapeutic effect, whether that be halting protein production or enhancing it.
To date, three children with Metachromatic Leukodystrophy and three others with Wiskott-Aldrich syndrome, both of which are inherited mutations, have been treated with HIV vectors. Their diseases have stopped progressing and some of the children have stopped showing symptoms for up to 32 months following therapy. 32
Another disease involving HIV vectors and showing promising results in the clinic is called X-linked adrenoleukodystrophy (X-ALD). It is a rare central nervous system disorder, most common in males, and often affects the brain, eventually producing a vegetative state. A lentiviral vector based on HIV has been used with positive benefits for two patients with this disease, while other vectors were not able to produce these results. X-ALD was first brought to the public eye in a 1992 movie called Lorenzo's Oil. 33
Gene therapy trials involving lentiviruses now account for about 3 percent of gene therapy trials worldwide. 34
Strategies
There will be a variety of activities so that needs of students with various learning styles are included. The teaching strategies used will be based on a scaffold model, where the initial introduction to the topic of AIDS and HIV will be more of an inquiry-based discussion than a lecture, starting with an open-ended question.
The initial discussions will be supplemented by several photographs and other information from the early years of the AIDS epidemic. I feel confident that with a topic as charged as this, I will have little trouble getting at least some students to share their knowledge and stories. This should get them and others invested in the topic through sharing their information with the rest of the class. The Think Share Pair approach could be good here, and I also like the Talking Stick approach, which mimics many patient support groups.
A student debate is an excellent way of getting students invested in learning, and it is exciting to see them perform in front of their peers. I would like to plan an activity using debate. Presentation is another good form of performance, and I want to employ it using a jigsaw method, where small groups of students talk as experts on a particular aspect of the broader subject. There will be one or two short research projects that I will expect each student to complete individually, for presentational preparation.
I want to use the Cooperative Learning model for the mathematical part of the unit, where the students develop their own science/mathematical models as a group.
Activities
Activity One
The initial discussion will take place on the first day of the lesson, and will be preceded by some still photographs that I will put up on the Smartboard. The famous cover of the November 1990 issue of LIFE magazine is one picture I would like my students to see, and I will supplement it with a few others, readily available on the web. The discussion will begin with an open-ended question that will ask the students if and how AIDS has affected their lives or the lives of their families and friends. I hope to eventually guide the discussion toward deeper understanding of the early years of the epidemic, before they were born, and what it was like for people who lived and died then, showing images from that time as the discussion proceeds. I want to record key words and memorable text from what we hear that day, and have these translated to a handout for student reference.
Activity Two
I have been involved in several trials in the Bay Area, and I am affiliated with SCOPE, a research organization in San Francisco that is funded in part by several drug companies, and is housed at San Francisco General Hospital, and operates in conjunction with the AIDS ward of the hospital. There is a strong possibility that I could get one of the researchers I know to talk to my class about what it is like to work in that field, the science, the politics, the money, and how AIDS has changed so much in the last thirty year. There is also the possibility that I could take the class on a field trip to the hospital, so they can get at least one picture of how a medical research team works. That might be fine with a small class.
Activity Three
As is detailed in the content section of the unit, there are several places in the life cycle of the HIV virus where science has successfully 'inhibited' its propagation with antiretroviral drug therapies. There are entry inhibitors (EI), reverse transcriptase inhibitors (RTI), integrase inhibitors (II), and protease inhibitors (PI), and then several different types of these. I would like to use the classic jigsaw strategy here, breaking the students into groups of three, four, or five, depending on class size, for the purpose of presenting to the class their particular piece of the puzzle which represents one step in the chain of events which inhibit the virus.
The day before the presentations, we will watch several animations on the Smartboard, the best one I found being 'Retrovirus Replication 3D Animation' on You Tube, and images from several other sites, some of which are listed in the last section of this paper. The students will have to do a small amount of research, with notes of some kind, to assist them in their presentation. They will be expected to answer questions posed by the other groups, and the interaction should strengthen their understanding, since each of the inhibitors have the same purpose, but accomplish it in different ways.
Activity Four
Jesse Gelsinger, a high school student, died from the result of a clinical trial involving gene therapy in 1999, at the age of eighteen. He suffered from a genetic liver disease, and was injected with an adenoviral vector carrying a corrective gene. He suffered a massive immune response triggered by the vector, and died of multiple organ failure and brain death several days later. His death represented a major setback for research in that field for several years afterwards. He is the first known death attributed to a gene therapy trial.
There will be a class discussion about what gene therapy is, and how it has also proven to be beneficial, especially for people who suffer from genetic diseases such as X-linked adrenoleukodystrophy, Metachromatic Leukodystrophy, and Wiskott-Aldrich syndrome, all mentioned in a previous section of this paper. This will be accompanied by still pictures, and several animations.
A debate on gene therapy will begin the following day, with students either signing up as pro or con, or as assigned by the instructor, or a combination of both. All students will be required to write and submit a short research paper about Jesse, and include other information they feel supports their pro or con stand on gene therapy trials. It will hopefully be a lively one.
Activity Five
This is a simple probability exercise using theoretical statistics about HIV infection. It can be done in class using a cooperative learning model with heterogeneous groups of students.
The HIV antibody test has been around since late 1984, and is now a relatively simple test, and results are back in minutes. It was a blood test originally, and results took up to two weeks to get back. The results show either a positive antibody response or a negative antibody response. However, it was possible, and it still is, to get either a false positive or a false negative result, although this is not likely.
Different data tables can be set up on a handout and distributed to the groups of students to work on in class, and compare, share, and explain their results. A population study is given, along with numbers of HIV+ and HIV- people, and positive and negative results for each group. An example table might look like this:
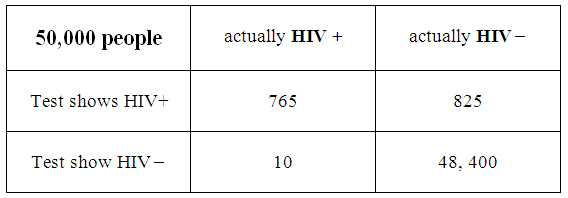
As can be seen from the table all four categories total to 50,000, and the students would then be asked to analyze the data given several prompts. These could have to do with the percentage of HIV+ people in the general population, the likelihood of getting false positives or false negatives, and the margin of error that can occur with either situation. As mentioned above, it would be a good idea to use several different data sets. A calculator may be used here, and each group will share its calculations and results with the class.
Activity Six
This is a more complex probability activity that involves viral mutation rates, and the ability of HIV to 'break out' of an established antiviral regimen, and restart its infection.
It has been established that the error rate of reverse transcriptase is very high when compared to cellular DNA, so there is always a chance of virus being released whose genome is sufficiently altered such that it becomes resistant to one or more of the antiviral drugs. Because there are four major events in the virus life cycle at which these drugs can stop the virus, and several different drugs which use different mechanisms to do that, this sets up a lot of possibilities for estimating the probability of a break out virus. This activity could be used in a different way than presented here. For instance, break out probabilities might be considered when only one inhibitor is being used rather than two, three, or all four, to simplify the activity. The following is the standard version.
Step One: determining viral load
Let's say we are a group of doctors and nurses working with an HIV patient who has never had any antiviral therapy. Our patient is producing 500,000,000 viruses a day that enter the blood, which would be typical of such a patient. Let's say this person is male, and the average male body contains 5 liters of blood. 'Viral load' is a measure of the amount of virus in a particular individual, and is defined as the number of viruses per milliliter of blood. Under these circumstances, what would the viral load be in our patient? Please give an answer and a short explanation of how you got it.
Step Two: Using entry inhibitors
Let's consider those viruses which are in the single milliliter of blood in the immediate vicinity of a group of cells. We will consider these to be viruses that can potentially enter some cell, which is the first step in infection. There are two different ways for the virus to enter and they are the two receptors on the cell surface called CCR5 and CXCR4 . Let's say that our patient is taking an antiviral that makes it difficult for the virus to interact with the CCR5 receptors, and 80% of the virus that come in contact with the cell cannot fuse with the cell using this receptor. What fraction (and percent) of the virus in this single milliliter of blood that could possibly enter the cell? How many virons does this fraction represent?
Let's now assume that the remaining virus which can use the CCR5 must also use the other receptor CXCR4 to finally enter the cell. Now let's now give our patient both antivirals, one for each receptor. The antiviral for the CXCR4 receptor will be effective in blocking about 40% of the viruses that were able to successful interact with the CCR5 receptors. Now what fraction and what percent of the virus that has successfully used both receptors in this single milliliter of blood will gain entry into a cell in this patient? How many viruses in this millimeter of blood will gain access to a cell?
Step Three: using reverse transcriptase inhibitors
There are two different types of reverse transcriptase inhibitors, nucleoside reverse transcriptase inhibitors (NRTI), and non-nucleoside reverse transcriptase inhibitors (NNRTI). NRTIs are competitive inhibitors which act as dummy nucleotides. They get inserted into the copy of the viral genome, and contain a defect which halts the copy process. They are therefore referred to as competitive inhibitors, since they compete with one of the four bases (Adenine, Thymine, Cytosine, and Guanine) in the growing chain of cDNA. NNRTIs inhibit the process externally by distorting positions of key elements in the process, and are thus referred to as non-competitive inhibitors. RNA contains a base called Uracil, which functions pretty much the same as Thymine.
So, let's give our patient one of the NRTIs. And let's further say that in order for our patient's virus to become resistant to this NRTI, it must be missing a sequence of 10 identical nucleotides that normally occur right in a row in one location on our patient's virus, TTTTTTTTTT, for example. The average HIV genome is 1,000 base pair long, and on average one error is made for every 1000 base pair, thereby creating a mutation, and making the cDNA molecule unrecognizable to the NRTI, and therefore resistant to it. What is the probability that this error will occur along this particular sequence of ten bases? Considering how many viruses were capable of entering cells, combined with the probability of NRTI resistance, how many viruses in our milliliter of blood still have the potential to complete the viral life cycle?
If we also give our patient an NNRTI, this will also further inhibit viral replication. Let's say that NNTRIs are successful at distorting 20% of the mutated viruses which are resistant to the NTRIs. What is the percent chance that a strain will be produced that will be resistant to both the NTRIs and the NNTRI from the pool of virus that entered a cell? How many virus are still a potential threat to our patient?
Step Four: using integrase inhibitors
The remaining viruses which have entered a cell, and gotten past the RTIs, now move into the nucleus to be integrated into our patient's DNA. We can treat our patient with an integrase inhibitor, which will prevent this from happening, at least in most cases. With that in mind, let's say that in order for our patient's virus to override the inhibitor, it must be missing a sequence of AGCT that occurs randomly at some place in the viral genome that the inhibitor recognizes. On average, and to the nearest whole number, how many times would we expect this sequence to repeat on the 1000 base pair long HIV genome? The number of viruses that resisted the integrase inhibitor can be calculated by dividing the number of viruses that entered the nucleus by the number you just found. How many viruses were able to integrate themselves into our patient's DNA?
Step Five: using protease inhibitors
The final opportunity to interfere with viral production is when the replicated viral genomes need to be segmented for the purpose of being packaged and exiting the cell. This is where the protease inhibitors come into play. For a virus to become resistant to and bypass the protease inhibitor, it must be missing a certain sequence in the location where the inhibitor binds, that occurs, on average, in one out of every twelve of the remaining viruses. These are the totally resistant viruses that will be packaged and exit the cell to possibly infect other cells in our patient. Of the 500,000,000 viruses we started with, how many remain that will exit the cell into our patient's body as totally resistant virus?
What might you speculate about this person's short and long term survival, considering your final result?
Activity 7
Finally, there will be a test on probability in Algebra II, and several questions about HIV will appear there, integrating math and science, as was my intention from the very beginning.
Appendix
The following two California Standards for Algebra II address several different kinds of probability.
18.0 Students use fundamental counting principles to compute combinations and permutations.
19.0 Students use combinations and permutations to compute probabilities.
In addition to the California Standards, our school district is putting a lot of focus this year on the new Common Core Standards. Several development days are already set aside for staff instruction and implementation. There are six standards for probability and statistics listed on the Common Core State Standards Initiative website. They are presented below.
CCSS.Math.Content.HSS-IC.A.1 Understand statistics as a process for making inferences about population parameters based on a random sample from that population.
CCSS.Math.Content.HSS-IC.A.2 Decide if a specified model is consistent with results from a given data-generating process, e.g., using simulation.
CCSS.Math.Content.HSS-IC.B.3 Recognize the purposes of and differences among sample surveys, experiments, and observational studies; explain how randomization relates to each.
CCSS.Math.Content.HSS-IC.B.4 Use data from a sample survey to estimate a population mean or proportion; develop a margin of error through the use of simulation models for random sampling.
CCSS.Math.Content.HSS-IC.B.5 Use data from a randomized experiment to compare two treatments; use simulations to decide if differences between parameters are significant.
CCSS.Math.Content.HSS-IC.B.6 Evaluate reports based on data.
Notes
1. Frederic D. Bushman. "Retroviral Integration and Human Gene Therapy." Journal of Clinical Investigation 117.8 (2007): p 2083
2. Victoria Angela Harden. AIDS at 30: a history. Washington, D.C.: Potomac Books, 2012. p. 5
3. HIV Prevention http://hivprevetion.blogspot.com/2011/03/april-24-san-francisco-resident-ken.html
4. Randy Shilts. And the Band Played On. NewYork: Souvenir Press, 2011. p. 439
5. Victoria Angela Harden. AIDS at 30: a history. Washington, D.C.: Potomac Books, 2012. p. 15
6. Ronald Bayer and Gerald M. Oppenheimer. AIDS doctors: voices from the epidemic. Oxford: Oxford University Press, 2000. p. 20
7. Victoria Angela Harden. AIDS at 30: a history. Washington, D.C.: Potomac Books, 2012. p. 23
8. Robert C. Gallo Virus hunting: AIDS, cancer, and the human retrovirus : a story of scientific discovery. New York, NY: BasicBooks, 1991. p. 147
9. Victoria Angela Harden. AIDS at 30: a history. Washington, D.C.: Potomac Books, 2012. p. 60
10. Ibid p. 67, 68, 69
11. Lauren Sompayrac. How pathogenic viruses think: making sense of virology. 2nd ed. Burlington, MA: Jones & Bartlett Learning, 2013. p. 4
12. Ibid p. 3
13.Ibid p. 17
14. Robert C. Gallo. Virus hunting: AIDS, cancer, and the human retrovirus : a story of scientific discovery. New York, NY: BasicBooks, 1991. p. 4
15. Ibid p. 17 p.104
16. Lauren Sompayrac. How pathogenic viruses think: making sense of virology. 2nd ed. Burlington, MA: Jones & Bartlett Learning, 2013. p. 87
17. Ibid p. 89
18. Ibid p. 90
19. Arnold J. Levine. Viruses. New York: Scientific American Library, 1992. p. 141
20. Gary L. Buchschacher. Lentiviral vector systems for gene transfer. Georgetown, TX: Eurekah.com :, 2003. p. 2
21. Robert C. Gallo, and Gilbert Jay. The Human retroviruses. San Diego: Academic Press, 1991. p. 54
22. Lauren Sompayrac. How pathogenic viruses think: making sense of virology. 2nd ed. Burlington, MA: Jones & Bartlett Learning, 2013. p. 145
23. " Maraviroc (Selzentry) , HIV/AIDS Drug , Patient , AIDSinfo." <http://aidsinfo.nih.gov/drugs/408/maraviroc/0
24. Baldwin, C. E., R. W. Sanders, Y. Deng, S. Jurriaans, J. M. Lange, M. Lu, and B. Berkhout. "Emergence Of A Drug-Dependent Human Immunodeficiency Virus Type 1 Variant During Therapy With The T20 Fusion Inhibitor." Journal of Virology 78.22 (2004): 12428-12437.
25. Lauren Sompayrac. How pathogenic viruses think: making sense of virology. 2nd ed. Burlington, MA: Jones & Bartlett Learning, 2013. p. 143
26. "Wolters Kluwer Health." LWW Journals - Beginning with A. <http://journals.lww.com/aidsonline/Abstract/2002
27. " Etravirine (Intelence) , HIV/AIDS Drug , Patient , AIDSinfo." <http://aidsinfo.nih.gov/drugs/398/etravirine/0/
28. " Raltegravir (Isentress) , HIV/AIDS Drug , Patient , AIDSinfo." <http://aidsinfo.nih.gov/drugs/420/raltegravir/0/professional
29. " Saquinavir (Invirase) , HIV/AIDS Drug , Patient , AIDSinfo." <http://aidsinfo.nih.gov/drugs/164/saquinavir
30. Gary L. Buchschacher. Lentiviral vector systems for gene transfer. Georgetown, TX: Eurekah.com :, 2003. p. 51
31. http://biology.kenyon.edu/slonc/gene-web/Lentiviral/Lentivi2.html#What
32. http://www.cbsnews.com/8301-204_162-57593387/gene-therapy-using-part-of-hiv-virus-treats-two-rare-childhood-diseases/
33. Samantha L. Ginn, Ian E. Alexander, Michael L. Edelstein, Mohammad R. Abedi, Jo Wixon. "Gene therapy clinical trials worldwide to 2012 – an update." The Journal of Gene Medicine 2013. p.66
34. Edelstein, Michael L., Mohammad R. Abedi, and Jo Wixon. "Gene Therapy Clinical Trials Worldwide" - Updated July 2013." The Journal of Gene Medicine 9.10 (2007): 833-842
Bibliography
Levine, Arnold J. Viruses. New York: Scientific American Library :, 1992.
Sompayrac, Lauren, and Lauren Sompayrac. How pathogenic viruses think: making sense of virology. 2nd ed. Burlington, MA: Jones & Bartlett Learning, 2013.
Harden, Victoria Angela. AIDS at 30: a history. Washington, D.C.: Potomac Books, 2012.
Gallo, Robert C. Virus hunting: AIDS, cancer, and the human retrovirus: a story of scientific discovery. New York, NY: BasicBooks:, 1991.
Bayer, Ronald, and Gerald M. Oppenheimer. AIDS doctors: voices from the epidemic. Oxford: Oxford University Press, 2000.
Shilts, Randy. And the Band Played On Politics, People and the AIDS Epidemic. New York: Souvenir Press, 2011.
Buchschacher, Gary L.. Lentiviral vector systems for gene transfer. Georgetown, TX: Eurekah.com :, 2003.
Gallo, Robert C., and Gilbert Jay. The Human retroviruses. San Diego: Academic Press, 1991.
Bushman, Frederic D.. "Retroviral Integration and Human Gene Therapy." Journal of Clinical Investigation 117.8 (2007): p 2083
Baldwin, C. E., R. W. Sanders, Y. Deng, S. Jurriaans, J. M. Lange, M. Lu, and B.
Berkhout. "Emergence Of A Drug-Dependent Human Immunodeficiency Virus Type
1 Variant During Therapy With The T20 Fusion Inhibitor." Journal of Virology 78.22
(2004): 12428-12437.
Edelstein, Michael L., Mohammad R. Abedi, and Jo Wixon. "Gene Therapy Clinical
Trials Worldwide" - Updated July 2013." The Journal of Gene Medicine 9.10 (2007):
833-842
Samantha L. Ginn, Ian E. Alexander, Michael L. Edelstein, Mohammad R. Abedi, Jo
Wixon. "Gene therapy clinical trials worldwide to 2012 – an update." The Journal of
Gene Medicine 2013. p.66
"Wolters Kluwer Health." LWW Journals - Beginning with A.
<http://journals.lww.com/aidsonline/Abstract/2002
" Maraviroc (Selzentry) , HIV/AIDS Drug , Patient , AIDSinfo."
<http://aidsinfo.nih.gov/drugs/408/maraviroc/0
" Etravirine (Intelence) , HIV/AIDS Drug , Patient , AIDSinfo."
<http://aidsinfo.nih.gov/drugs/398/etravirine/0/
" Raltegravir (Isentress) , HIV/AIDS Drug , Patient , AIDSinfo."
<http://aidsinfo.nih.gov/drugs/420/raltegravir/0/professional
" Saquinavir (Invirase) , HIV/AIDS Drug , Patient , AIDSinfo."
<http://aidsinfo.nih.gov/drugs/164/saquinavir
<http://www.cbsnews.com/8301-204_162-57593387/gene-therapy-using-part-of-hiv-
virus-treats-two-rare-childhood-diseases/
Lentiviral Vectors Page 2 - Biology
<http://biology.kenyon.edu/slonc/gene-web/Lentiviral/Lentivi2.html#What
HIV Prevention
http://hivprevetion.blogspot.com/2011/03/april-24-san-francisco-resident-ken.html
Additional Reading and Resources
Nicholl, Desmond S. T.. An introduction to genetic engineering. 3rd ed. Cambridge: Cambridge University Press, 2008.
Scott, Andrew. Pirates of the cell: the story of viruses from molecule to microbe. Oxford Oxfordshire: Basil Blackwell, 1985.
Leibowitch, Jacques. A strange virus of unknown origin. New York: Ballantine Books, 1985.
Gallo, Robert C., Myron Essex, and Ludwik Gross. Human T-cell leukemia/lymphoma virus: the family of human T-lymphotropic retroviruses, their role in malignancies and association with AIDS. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory, 1984
Gallo, Robert C., and Flossie Staal. Retrovirus biology and human disease. New York: Dekker, 1990.
" HIV Replication Cycle." National Institute of Allergy and Infectious Diseases (NIAID). N.p., n.d. Web. 28 June 2013. <http://www.niaid.nih.gov/topics/HIVAIDS/Understanding
How the HIV Infection Cycle Works
<https://highered.mcgraw-hill.com/sites/0072495855/student_view0/chapter24/animation__how_the_hiv_infection_cycle_works.html
"Retrovirus Replication 3D Animation - YouTube." YouTube. N.p., n.d. Web. 28 June 2013. <http://www.youtube.com/watch?v=HhhRQ4t95OI>.
"Retrovirus Replication." Access Excellence @ the National Health Museum. N.p., n.d. Web. 28 June 2013. <http://www.accessexcellence.org/RC/VL/GG
LOS Computational Biology: Retroviral Integration Process in the Human Genome: Is It Really Non-Random? A New Statistical Approach." PLOS Computational Biology: A Peer-Reviewed Open-Access Journal. N.p., n.d. Web. 28 June 2013. <http://www.ploscompbiol.org/article/info%3Adoi%2F10.1371%2Fjournal.pcbi.1000144>.
"Genetic Vaccines and Therapy , Full text , The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery." Genetic Vaccines and Therapy . N.p., n.d. Web. 28 June 2013. <http://www.gvt-journal.com/content/2/1/9>.
Comments (0)
THANK YOU — your feedback is very important to us! Give Feedback
