- Login
- Home
- About the Initiative
-
Curricular Resources
- Topical Index of Curriculum Units
- View Topical Index of Curriculum Units
- Search Curricular Resources
- View Volumes of Curriculum Units from National Seminars
- Find Curriculum Units Written in Seminars Led by Yale Faculty
- Find Curriculum Units Written by Teachers in National Seminars
- Browse Curriculum Units Developed in Teachers Institutes
- On Common Ground
- Publications
- League of Institutes
- Video Programs
- Contact
Have a suggestion to improve this page?
To leave a general comment about our Web site, please click here
The Chemistry of Weather
byDeborah James-JohnsonIntroduction
My unit topic is to explore and explain the chemistry behind weather. Elementary teachers are faced with teaching students at a very early age about the weather. After all, weather is what prompts one into making decisions, or the parents, into what to wear each day. I remember teaching my second graders about weather. We took daily temperature readings, drew pictures showing the amount, or lack thereof, of cloud cover in the sky. If it rained, we put out buckets and measured and recorded how much rain fell in a certain time period. We even made flags and went out and observed the wind direction and strength of the wind. All that I was teaching my students; I had no idea of the chemistry behind the weather. When I think of chemistry, I think of chemicals, Bunsen burners, goggles, and rubber gloves. I didn't see how weather and chemistry related. I know that there's chemistry everywhere and this is an attempt to relate weather to basic chemistry.
Rationale
This unit will be taught to four sixth grade general science classes at Betsy Ross Arts Magnet School in New Haven, CT., which is an inner-city school district. Betsy Ross is a true magnet school that attracts students from a variety of surrounding suburbs in Connecticut due to the strong arts program; therefore, we have a diverse population of students. Science is departmentalized at Betsy Ross and each class period is fifty-two minutes long. The sixth grade curriculum has four science standards that must be taught throughout the year and these are: Ecosystems, Simple Machines, How Human Activities Affect Our Waters, and Weather.
The subject of weather is the unit that I will develop my curriculum unit on. I teach about air pressure, seasonal changes, global warming, the water cycle, etc. What I intend to accomplish in this unit is to explain these weather topics in a language of chemistry, not necessarily for my students, but for other teachers that may have to teach weather on an elementary level and want to know the true science behind it. For years, I have been made aware that many elementary teachers are somewhat intimidated by science and avoid teaching it to their students. My attempt is to take the "scary" out of chemical equations and make it more comfortable for me to teach and have confidence and to hope that others will benefit from this information, as well.
Background
Weather is the state of the air at a particular place and time. Weather can be warm or cold, dry or wet, windy or cloudy. To understand how these phenomena occur, we must first start with understanding matter. Matter is anything that takes up space and has mass. Matter exists on Earth in the forms of solids, liquids and gases. What defines the difference between a solid, liquid, and a gas is the way in which the particles that make up the matter are arranged and behave. All matter has particles that are in constant motion and this is based on the kinetic-molecular theory. In a solid, particles are arranged closely packed and these particles vibrate and move around a fixed point. Solids have a definite shape and volume. Examples of solids on Earth are glaciers, ice, snow caps, and icebergs.
As heat is applied to the solid, the particles move farther apart and are in constant motion which makes the liquid to be fluid. A fluid is a substance that can flow; thus, liquids take the shape of its container. Liquids take up the space of its container and have a definite volume. One example of a liquid on Earth can be found in salt and fresh water bodies, such as oceans, lakes, rivers, and ponds. Particles in a liquid move slower and are closer together than those in a gas.
Gases consist of particles that are fast-moving that are far apart. Gases are fluid, as liquids are fluid. Gases do not have a definite volume and take up the space of a container. The particles move rapidly and bounce off one another, like hard spheres. The kinetic molecular theory applies only to ideal gases. "An ideal gas is an imaginary gas that perfectly fits all the assumptions of the kinetic-molecular theory. The fact is that on Earth there are real gases which are gases that do not behave according to the kinetic-molecular theory; thus, the particles collide and may stick together instead of bouncing off one another, as they would do in an ideal gas situation". 1 An example of gases that exist on Earth as it relates to weather is the atmosphere.
The atmosphere consists of four layers: the troposphere, the stratosphere, the mesosphere, and the thermosphere. The thermosphere is divided into the ionosphere, and the exosphere. These layers are classified by the changes in temperature. Weather occurs in the troposphere. Tropo- means turning or changing. As the altitude in the troposphere increases, the temperature decreases; thus, snow peaks on the tops of mountains. Earth's atmosphere consists of nitrogen at 78 percent, oxygen at 21 percent, and other gases at 1 percent. The other gases include carbon dioxide; water vapor is also present but not included in these percentages because its amount varies. There are other components, as well, such as particles of liquids and solids.
There are four measurable quantities that express a gas: volume, temperature, number of molecules, and pressure. There are gas laws that express these four properties of gases. As it relates to weather, there are four laws that will be examined: Boyle's law, Charles law, Gay-Lussac's law, and Dalton's law. "The gas laws are simple mathematical relationships between the volume, temperature, pressure, and the amount of a gas. Pressure is the result of molecules making collisions against a container's wall." 2
"Boyle's law states that the volume (V) of a fixed mass of gas varies inversely with the pressure at constant temperature (T)." 3 It is expressed as V = k/P or PV = k, where k is a constant.
This means that if the temperature stays the same and you decrease the pressure on the gas, the volume will increase. We see this on Earth when you climb a mountain. As you go up, the air particles are more spread out; thus, the pressure is reduced. That is why people feel light-headed at higher elevations because there are fewer air molecules per square centimeter.
With meteorology, which is the study of weather, weather balloons are used to forecast weather. Why do balloons rise? This is due to the next law, Charles's law. "Charles's law states that the volume of a fixed mass of a gas at constant pressure varies directly with the Kelvin temperature. Kelvin temperature is a scale that starts at -273.15°C." 4 It is expressed as V = kT or V/T = k, where T = Kelvin temperature. This means if the pressure stays the same and you increase the temperature, then the volume will increase. We see this on Earth with hot air balloons. As heat is applied, the balloon inflates. This goes back to the property of gases that the more heat makes particles move faster and can also change states of matter. This causes ice to melt and water to evaporate which explains part of the water cycle. Remember, particles in solids only vibrate; particles in liquids move quicker and the particles are not as close; and then particles in gases move faster and are spread more apart.
The third law that relates to weather is the Gay-Lussac's law. This law states: "the pressure of a fixed mass of gas at a constant volume varies directly with the Kelvin temperature." 5 It is expressed as P = kT or P/T = k, where k is a constant. This means if the temperature increases, the pressure increases, as well. Therefore, as air molecules are heated, they move faster, thus making more collisions and pressure is increased. The language used by meteorologists is high and low atmospheric or air pressure. Air pressure is the phenomenon of a column of air pushing down on a particular area. Air pressure not only pushes downward but it is the weight of air pushing in all directions. The atmosphere closest to Earth has more pressure than the atmosphere at higher altitudes because if you think if a column of air pushing down on an object, there is all the weight of the air nearest to the surface of the Earth. The farther you go up in elevation, the less air is there to add to the weight.
Air has properties which are mass and density, as well as pressure. The density of the atmosphere must be discussed, at this point. Density is equal to mass divided by volume, d = m/V. Since air has density, the denser the air is, the more pressure there is. In other words, denser air has more mass per unit volume than less dense air. Density is affected by three factors: the amount of water vapor, elevation and temperature. Warmer air is less dense than colder air and warmer air holds more water vapor than colder air. Therefore, hot air with more water vapor is less dense and has lower air pressure. But, you may think that if air has more water vapor in it, that the air would be denser. This is where Dalton's law helps to explain this.
Dalton's law deals with the pressure of a gas mixture which is the sum of each individual gas pressure. We know that our atmosphere is a mixture of gases as stated above. Water vapor is less dense than nitrogen or oxygen of the other gases mentioned. "Dalton's law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases." 6 This law is expressed as: P T = P 1 + P 2 + P 3 + …, where P Tis the total pressure of the mixture, and P 1, P 2, P 3, … are the partial pressures of the component gases. Water vapor has a lighter molecular mass than diatomic nitrogen and diatomic oxygen, which are found in our troposphere. Water vapor has two hydrogen molecules and an oxygen molecule giving it an atomic mass of 18. Diatomic nitrogen has an atomic mass of 28 and diatomic oxygen has an atomic mass of 32. So, although air with water vapor feels heavier and is harder to breathe, it is still less dense than cold, drier air. Water vapor is a greenhouse gas that traps heat on Earth, as well.
Water vapor contributes about 60% to the warming process of the Earth's atmosphere. It is like a sweater surrounding the Earth, trapping the heat radiation from the ground. Energy is stored in water vapor as heat. Water vapor is considered to be a positive feedback to greenhouse warming meaning as more water is evaporated into the air due to higher temperatures, the warmer the air will become, thus making a positive feedback loop. However, along with more evaporation of water into the air leads to more cloud formation which can lead to the clouds reflecting the Sun's radiation away from the Earth. The cloud cover lowers the temperature, thus producing a negative feedback loop and increased water vapor could have a self-correcting effect. There is much debate, currently, about this issue of water vapor and its impact on global warming, which is the gradual increase of the Earth's atmospheric temperature.
Cloud formation is due to a phase change called condensation. Just the opposite of evaporation, condensation occurs when heat is released from a gas. Clouds are formed when water vapor condenses around tiny solid particles in the air. Clouds are classified by their appearance and height. The three basic forms of clouds are stratus, cirrus, and cumulus. Cirrus clouds are high in the sky and are white, thin, and wispy. The cumulus clouds are puffy with a flat base and look dome-like resembling cauliflower. The stratus clouds look like sheets that cover the sky. The height of clouds classifies them further. High clouds are usually white and made up of ice crystals. Since there is more water vapor available at lower altitudes, low and middle altitude clouds tend to be darker and denser. The reason why not all clouds precipitate, although they all contain water, is that the size of droplets are so small that for high clouds, when the droplets fall, even in humid air, they evaporate before they reach the ground. A raindrop that reaches the ground is about a million times larger than a cloud droplet. For a rain droplet to form, these cloud droplets must join together in order to fall to Earth. This phenomenon is called the Bergeron process and the collision-coalescence process.
The Bergeron process relies on two peculiar properties of water. One is that pure water suspended in air does not freeze until it hits -40°C. This is called being supercooled. "Supercooled water will readily freeze if it is sufficiently agitated. This explains why airplanes collect ice when they pass through a liquid cloud made up of supercooled cloud droplets. Also, supercooled cloud droplets will freeze around solid particles that have a crystalline form. These particles are named freezing nuclei and have given rise to the technology of cloud seeding." 7
The second property, saturation, states that, "when air is saturated (100 percent relative humidity) with respect to water, it is supersaturated (relative humidity greater than 100 percent) with respect to ice. Therefore, when the relative humidity is 100 percent with respect to water, then the relative humidity, with respect to ice is nearly 110 percent." 8 Ice crystals cannot coexist with water droplets and eat up the excess water vapor; this lowers the relative humidity near the droplets and the droplets then evaporate making the ice crystals grow larger. They grow large rapidly and then fall; on their descent, cloud droplets adhere to these ice crystals. This chain reaction continues until larger crystals form producing snow crystals. The reason why snowflakes have a hexagonal shape is that the water molecules bind together to form a hexagonal shape and the snowflake is formed on particles called freezing nuclei. Therefore, even in summer months, snowflakes have formed, but melt before they reach the Earth's surface as rain droplets.
The spherical shape of rain droplets is due to another property of water called surface tension. "Surface tension is a force that tends to pull adjacent parts of a liquid's surface together, thereby decreasing the surface area to the smallest possible size. Surface tension is the attractive force between particles in a liquid." 9 Water has a higher surface tension than most liquids because of the hydrogen bonding between water molecules.
Another, less obvious property of water is capillary action whereby the same properties of water that exist in surface tension exist here, as well, and that is the strong attraction between water molecules. Capillary action is the attraction of the surface of a liquid to the surface of a solid. This can be evidenced by roots of plants taking up water to its leaves. How this fits into weather is that trees transpire, or give off some of this water to the atmosphere.
Still another property of water is specific heat. Specific heat is defined as the amount of heat needed to raise 1 gram of a substance 1°C at sea-level atmospheric pressure. Water has a relatively high specific heat. This can explain why water takes much more heat to raise its temperature than land. There are other factors to explain the heat differential of water versus land. Water moves around where as land does not. This will cause water to heat more slowly than land. Another reason is that heat does not penetrate land deeply; therefore, only a thin layer above the land is heated. This is called conduction. Conduction is heat that is transferred from the ground to the air. Molecularly speaking, conduction is the transfer of heat through matter by molecular activity. On land, there is a thin layer from the ground to the air above it that is being heated. Since water is moving, a much thicker layer of water is heated to just moderate temperatures. That is why it is much more desirable to be near water in the hot summer months and the same holds true in the cold winter months. Land cools much more quickly because of the thin layer and since land is not mobile as water, land cools much more quickly than water. Also, the cooler water at the surface sinks and displaces the warmer water underneath; therefore, a larger mass of water will be cooled. Since air is a poor conductor of heat, conduction is only important to the area of the ground; the air directly above the ground is, therefore, considered the least significant factor in heat transfer.
Radiation plays a significant role in the transfer of heat from the land-sea surface to the atmosphere and vice versa. The heat that is gained by the lowest level of the atmosphere through radiation or conduction is usually transferred by convection. "Convection is the transfer of heat by the movement of a mass or substance from one place to another. It can only take place in liquids and gases." 1 0
Relating specific heat and humidity, water vapor stores heat because it took energy to evaporate liquid water. This will explain why in the desert there are relatively colder nights to warmer days opposed to an area that is near a large body of water where increased evaporation can exist. Another factor that contributes to the differences in heating of land and water is that land is opaque and heat is absorbed only at the surface, whereas water is transparent and allows more solar radiation to penetrate at a deeper level. Another factor is that evaporation, which is a cooling process, happens more from bodies of water than that from land. All of these factors help to explain why water warms more slowly and cools more slowly than land.
The chemistry behind evaporation acting as a cooling agent is that it takes energy to evaporate and that energy comes from heat energy, thus making it feels cooler. Perspiration uses this principle. When you perspire and the perspiration evaporates, you feel cooler. Conversely, condensation releases heat energy. This energy helps to produce violent weather and can transfer a lot of heat energy from the tropical region to the polar region.
Condensation and evaporation are examples of phase changes, changing form one state of matter to another; this occurs through either the addition or the reduction of heat, but there are more phases such as freezing, which was discussed earlier in the supercooling of water droplets forming snowflakes. Then there is melting, where given enough heat energy, solid changes to a liquid and heat is being absorbed, thus creating a cooling effect like that of evaporation. Lastly, there is sublimation which is when a gas is converted directly to a solid and vice versa and the liquid form is skipped. Water vapor converting directly to a solid can be seen as frost and snowflakes. This is also known as deposition. Ice converting directly to water vapor can be seen when snow banks and ice seem to disappear. These phase changes are what drives the hydrologic cycle (water cycle) of the Earth since water from the oceans continuously is evaporating, reaching such heights as to condense forming clouds that eventually precipitate, which is in the form of either snow, rain, sleet, or hail.
Water vapor in the air is termed as humidity. Humidity is the amount of water vapor in the atmosphere. Humidity can be quantitatively expressed as humidity, absolute humidity, specific humidity, and the most common in meteorology, as relative humidity. "Absolute humidity is stated as the weight of water vapor in a given volume of air (usually as grams per cubic meter). As air moves from one place to another, even without change in moisture content, changes in pressure and temperature causes changes in volume and consequently the absolute humidity, thus limiting the usefulness of this index." 1 1 This is why meteorologists use specific humidity to express water vapor content in the atmosphere. "Specific humidity is expressed as the weight of water vapor per weight of a chosen mass of air, including the water vapor." 1 2 Since it is measured in units of weight (usually grams per kilogram), specific humidity is not affected by changes in pressure or temperature. "Relative humidity is the ratio of the air's water vapor content to its water vapor capacity at a given temperature." 1 3 Therefore, on days where the air is saturated, there is 100 percent relative humidity. Moisture being added to the air through evaporation will increase the relative humidity.
Dew point temperature is another important concept related to relative humidity. 1 4 Dew point is the temperature to which a parcel of air would have to be cooled in order to reach saturation. Simply, dew point is the temperature at which condensation begins. If the dew point is above freezing, the water vapor will change to water droplets. If the dew point is below freezing, the water vapor may change to ice crystals. For condensation to occur, a surface must be provided for this condensation to adhere to. In the case of dew, objects near or on the ground provide the needed surface. High in the atmosphere, tiny particles serve as condensation nuclei and provide the surfaces needed for the condensation of water vapor to form clouds. Without these condensation nuclei, "a relative humidity of 400 percent would have to be reached in order for clouds to be produced." 1
Strategies/Activities
Students will conduct several activities to demonstrate weather phenomena and make connections to the chemistry aspect of those activities. In incorporating the strategies using their interactive notebooks, students will keep all record-keeping, handouts, class work, and homework in their notebooks in an organized and prescribed fashion. The interactive notebook helps students retain information because it makes them revisit their notes by having them write questions, summarizing, and completing a creative aspect while doing their homework. The interactive notebook is based on the Cornell-style of note taking where notes are on the right, questions are on the left, and a summary section is at the bottom of each page. The top of the page has the date and the objective of the day's lesson.
Through hands-on activities, students will see Boyle's law at work, as well as Charles's law, and Gay-Lussac's law to make chemistry connections to weather phenomena. Students will make certain weather gauging instruments and be able to use these devices as a way to forecast weather. Students will keep a class calendar documenting temperature, barometric pressure, humidity, and wind speed. They will make observations and record the amount of cloud cover and whether there was precipitation that day, and if so, what kind. Students will also keep a record of when the sun rises and sets, which will come by viewing the newspaper daily.
Activity One
Students will make a bottle-balloon apparatus and heat, and then cool it and record results. (See Appendix for more details). Students will be introduced to the physical change of matter by changing the temperature, which will have a direct impact on pressure and volume of this closed system. Students will be able to relate this activity to what happens on Earth when temperature increases and how it affects gas and water molecules. They will see the connection of why clouds are usually up in the sky.
Activity Two
Students will make a rain gauge to begin recording the amount of precipitation on a daily basis on a data table and, after one week, will graph their results. (See Appendix for more details).
Activity Three
Students will discover that air has density by conducting an experiment using a deflated balloon and massing it, then blowing air into the balloon and massing it to see the change in mass. (See Appendix for more details).
Activity Four
Students will investigate how pressure and volume are related. They will see that, with an increase in pressure, volume will decrease. They will be introduced to Boyle's gas law that states that when pressure is applied to a certain amount of gas, that volume is inversely affected. Boyle's law: P 1V 1 = P 2V 2 . Students will use syringes to experience pressure and volume. (See Appendix for more details).
Activity Five
Students will perform the "egg in a bottle" experiment to understand Gay-Lussac's law which states that the pressure of a given amount of gas is directly proportional to temperature, if the volume stays the same. It is expressed as: P 1/P 2 = T 1/T 2. (See Appendix for more details).
Activity Six
Students will be introduced to the Kelvin scale and the idea of absolute zero by looking at the ratio of volume to temperature. Charles's law states: V = kT (where k is a constant) or, V 1/T 1 = V 2/T 2. Students will be given scenarios whereby they will calculate the volume of balloons if temperature changes. (See Appendix for more details).
Activity Seven
Students will explore how the ability to absorb heat and retain heat differs among different materials. This exercise will make students aware how land and water differ in their ability to store heat. This will teach them the concepts of radiation, conduction and convection. (See Appendix for more details).
Activity Eight
Students will learn about water in the atmosphere by modeling how fog forms. They will learn what humidity is and how it is measured and categorize three main cloud types. They will use a two-liter bottle, hot water and ice to simulate fog formation. (See Appendix for more details).
1 Raymond H. Davis, et al, Modern Chemistry.: Holt, Rinehart and Winston, 2002, p. 303
2 Ibid, p. 313
3 Ibid, p. 313
4 Ibid, p. 317
5 Ibid, p. 319
6 Ibid, p. 322
7 Frederick K. Lutgens and Edward J. Tarbuck, The Atmosphere: An Introduction to Meteorology. Prentice-Hall, 1979, p. 102
8 Ibid, p. 102
9 Raymond E. Davis, et al., Modern Chemistry, Holt, Rinehart, and Winston, 2002, p. 365
10 Frederick K. Lutgens and Edward J. Tarbuck, The Atmosphere: An Introduction to Meteorology. Prentice-Hall, 1979, p. 30
11 Ibid, pp. 69-70
12 Ibid, p. 70
13 Ibid, p. 70
14 Ibid, pp. 70-71
15 Ibid, p. 76
Works Cited
Battan, Louis J. Fundamentals of meteorology. 2nd ed. Englewood Cliffs, N.J.: Prentice-Hall, 1984.
This book is an introduction to meteorology and examines how the atmosphere affects humans. The reader will have some basic knowledge of the atmosphere, what scientist know about it now and questions that still have to be explored.
Cobb, Cathy, and Monty L. Fetterolf. The joy of chemistry: the amazing science of familiar things. Amherst, N.Y.: Prometheus Books, 2010.
This book attempts to take the fear out of chemistry and how everyday things can be explained to the nonscientist. It helps to explain our world and the phenomena we see daily.
Davis, Raymond E. Modern chemistry. Orlando: Holt, Rinehart and Winston, 2009.
This high School chemistry book gives basic facts and theoretical principles on chemistry and how chemistry relates to certain properties of weather.
Lutgens, Frederick K., and Edward J. Tarbuck. The atmosphere: an introduction to meteorology. Englewood Cliffs, N.J.: Prentice-Hall, 1979.
This book is written for students interested in the study of meteorology. It has many useful illustrations that help to explain weather phenomena.
Stacy, Angelica M., Janice A. Coonrod, and Jennifer Claesgens. Weather: gas laws and phase changes. Prelim. ed. Emeryville, CA: Key Curriculum Press, 2003.
This student workbook offers science inquiry activities that relate chemistry with weather phenomena.
Stepp, Richard D.. Making theories to explain the weather. Arcata, CA: Stepp (Humboldt State University, Arcata, CA 95521), 1983.
This book is not like the typical introduction to meteorology. It raises questions and engages the reader to do science rather than read science. This book is more of a narrative because it outlines trials and errors that had lead meteorologists to come to the present day conclusions.
Wayne, Richard P. Chemistry of atmospheres. 3rd ed. Oxford: Oxford University Press, 2000.
This book focuses on atmospheric chemistry and how humans have an impact on the lower level of the atmosphere and even that aircraft operations may affect the troposphere.
Chicago formatting by BibMe.org.
Appendix
6.2 - An ecosystem is composed of all the populations that are living in a certain space and the physical factors with which they interact.
Populations in ecosystems are affected by biotic factors, such as other populations, and abiotic factors, such as soil and water supply.
Populations in ecosystems can be categorized as producers, consumers and decomposers of organic matter.
C 4. Describe how abiotic factors, such as temperature, water and sunlight, affect the ability of plants to create their own food through photosynthesis.
C 5. Explain how populations are affected by predator-prey relationships.
C 6. Describe common food webs in different Connecticut ecosystems.
Science and Technology in Society – How do science and technology affect the quality of our lives? (EARTH)
6.4 - Water moving across and through earth materials carries with it the products of human activities.
Most precipitation that falls on Connecticut eventually reaches Long Island Sound.
C 10. Explain the role of septic and sewage systems on the quality of surface and ground water.
C 11. Explain how human activity may impact water resources in Connecticut, such as ponds, rivers and the Long Island Sound ecosystem.
Activity One Worksheet
Bottle-Balloon Apparatus Exercise
Materials for every two students:
-One Aquapod water bottle
-Medium-size balloon
-Pan of ice
Material for class:
-Hot plate
-500 ml glass beaker half filled with water
-Water
Procedure:
1.Place a small amount of water in water bottle
2.Stretch balloon over the mouth of the water bottle
3.Place bottle-balloon apparatus in heated glass beaker
4.Allow bottle-balloon apparatus to heat until water inside bottle evaporates
5.Record observations
6.Carefully remove bottle-balloon apparatus from heat and place it immediately in pan of ice with a little bit of water in pan.
7.Record observations.
Discussion Questions:
1.Describe and draw the motion of how the air molecules are moving in the bottle when it was heated.
2.Describe and draw how the water molecules are moving in the bottle when it was cooled.
3.Describe the motion of the air molecules that are closer to the ground on the Earth.
4.Describe the motion of the air molecules that are up in the mountains.
5.Summarize what happens to air molecules when they are heated and cooled.
Adapted from the University of New Haven Greater New Haven Collaborative
Activity Two
Students will make a rain gauge and keep a daily log of the amount of rain that fell in a given area.
Materials for every four students:
-Can or plastic container with a large mouth
-Centimeter paper
-Tape
-Scissors
Materials for the class:
-Laminating machine
Procedure by teacher:
a.Laminate centimeter paper (teacher)
b.Cut centimeter paper into one cm strips
Procedure by students:
a.Tape the centimeter strip on the inside of the can
b.Place can outside in a designated area
c.Keep a log and record the amount of rain that precipitated daily.
Activity Three
Students will mass air.
Materials:
-Triple beam scale
-Round balloons
Procedure:
a.Mass a deflated balloon and record.
b.Calibrate scale to have a zero reading with deflated balloon on it.
c.Blow air into the balloon and knot it closed
d.Place inflated balloon on scale and record the results.
Discussion:
If there was a difference between the deflated balloon and the inflated balloon, what explanation can you give as to what made that difference?
Activity Four
Students will discover that air has pressure.
Materials: (for each pair of students)
-50 mL plastic syringes (uncapped)
Procedure:
a.Cover the tip of the syringe with your fingertip
b.Record the volume of air in the syringe, using the scale on the syringe
c.Apply pressure to the syringe by pushing the plunger to read 40 mL.
d.Apply more pressure to the syringe, so that the inside volume reaches 30 mL.
e.Record your observations.
Answer the following:
1.What did you feel when you pushed the plunger down from 40 mL to 30 mL, to 20 mL?
2.Are you able to push the plunger down to the bottom? Explain why or why not.
3.Does the amount of air inside the syringe change? Explain your thinking.
(Adapted from Weather: gas laws and phase changes)
Activity Five
Materials
-A wide mouth juice bottle
-Hardboiled eggs
-Several strips of paper (2 x 6 inches)
-Matches
-Water
Procedure:
1.With adult supervision, light the strip of paper with a match and drop the lit paper inside the juice bottle.
2.When the strip of paper's fire extinquishes, place the hard-boiled egg over the mouth of the bottle.(It helps to use a llittle vegetalbe oil around the mouth of the bottle).
3.Wait until the egee slides into the bottle.
4.To reverse the procedure, turn bottle upside down and blow into the bottle. The egg will pop out into your mouth.
Activity Six
Students will calculate volumes of balloons when the temperature changes.
Materials:
1.Calculators
2.Paper and pencils
Procedure:
Copy and complete the following table into your notebook:
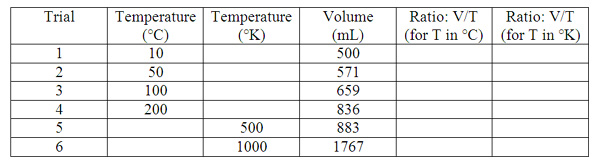
Answer the following questions:
1.What did you notice about the ratio of volume to temperature for the different trials?
2.When the temperature was doubled in degrees Celsius, did the volume also double?
3.When the temperature was doubled in degrees Kielvin, did the volume also double?
4.Whenever the volume doubled, did the temperature also double?
5.What seems to be the difference between using the Kelvin scale and the Celsius scale?
(Adapted from Weather: gas laws and phase changes)
Activity Seven
Student will observe that different materials absorb and retain heat differently.
Materials (for the class):
-Bag of black gravel (from pet store)
-Bag of white gravel
-Water
Materials for each pair of students:
-Three clear cups
-Three thermometers
-One heat lamp
-Timer
Procedure:
1.Fill each cup half full with black gravel, white gravel, and water
2.Place thermometer in each cup
3.Place all three cups under the heat lamp
4.Record the temperature of each cup every five minutes under the heat lamp
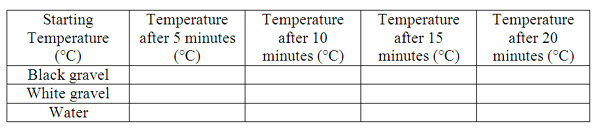
Record the temperature every five minutes after the cups are removed from the heat lamp.

Answer the following questions:
1.Which material absorbed the most heat?
2.Which material retained the most heat?
3.How can you relate this to why people are attracted to beachfront or waterfront properties?
4.Research the average temperatures in Kansas City, to that of New Haven, CT.
Activity Eight
Students will make a cloud form in a bottle.
Materials for group of 2-4 students:
-Two 2-liter bottle with cap
-Tap water
-Hot water
-Long matches
Procedure:
1.Place a small amount about 10 mL) of water in the 2-liter bottle.
2.Light a match, then blow it out and let the smoke go inside the bottle. Hold the bottle with the open end down.
3.Quickly screw the bottle top on the bottle.
4.Shake the bottle to allow the added moisture to mix with the air inside the bottle.
5.Squeeze the bottle and release it quickly. Repeat this several times. Observe what happens.
6.Repeat the same experiment, but this time put 10 mL of hot water (above 80°C) inside the bottle. Observe what happens.
7.Repeat the same experiment this time using a dry 2-liter bottle.
Answer the following questions:
1.What did you observe inside the bottle as you squeezed and released it?
2.Why did you shake the bottle in the beginning?
3.When the bottle was squeezed, how did this change the volume of the container?
4.When we released the bottle, how did this change the volume of the container?
5.What did you observe when you used the dry bottle?
6.Which bottle formed the largest cloud? Explain why.
Comments (0)
THANK YOU — your feedback is very important to us! Give Feedback
