- Login
- Home
- About the Initiative
-
Curricular Resources
- Topical Index of Curriculum Units
- View Topical Index of Curriculum Units
- Search Curricular Resources
- View Volumes of Curriculum Units from National Seminars
- Find Curriculum Units Written in Seminars Led by Yale Faculty
- Find Curriculum Units Written by Teachers in National Seminars
- Browse Curriculum Units Developed in Teachers Institutes
- On Common Ground
- Publications
- League of Institutes
- Video Programs
- Contact
Have a suggestion to improve this page?
To leave a general comment about our Web site, please click here
Caution! Drug Diffusion Underway: Using Inquiry to Understand How Drugs and the Body Interact
byValerie SchwarzIntroduction
Fourth grade students have a natural curiosity to learn about the world around them. This unit explores how people get medicine inside their body, how the medicine is distributed, metabolized and excreted. The unit focuses on scientific investigation and math as a means of teaching the students how pharmaceutical drugs are designed to last for a particular duration on time.
Student Demographics
The students I teach live in the city, but tend to come from middle class backgrounds; however, not all of the students at my school are so fortunate. There is a small population of students receiving free and reduced lunch, and a few homeless students as well. The economic spectrum is wide, as is the range of academic ability among a typical class. In fourth grade the reading levels typically span from first or second grade up to eighth grade or higher. Another important factor that can really separate and impact student achievement is the amount of support the students receive at home. At my school the large majority of students have very involved parents who are engaged when it comes to assisting their child with homework or studying. These parents have high expectations and want to make sure their child is sufficiently challenged. However, there are usually a few students with little if any academic support at home. I also typically have several students with special needs in my class, who receive additional support from a special education teacher. Given the wide range of students, I find it is important to carefully scaffold the lessons and present the material in a variety of ways so that all students can be successful and access the curriculum.
Objectives
My fourth graders have objectives to master. The Virginia state objectives I will address in this unit are scientific investigation, measurement, fractions, data collection and elapsed time. These topics will be embedded throughout the curriculum unit. Standard 4.1L says that students will construct a physical model to clarify an explanation, demonstrate a relationship or solve a need. Standard 4.5 Students need to understand that organisms have structural adaptations or physical attributes that help them meet a life need. The unit will use the human body and the structural adaptations or physical attributes of different cells and tissues to address the 4.5 standard.
Rationale
Have you ever walked down the medicine aisle in the grocery store? Some medicines advertise that they last 12 hours. Others are taken every four or six hours. Have you ever wondered why? The topic of drugs and how they work is relevant to our lives. Everyone has been sick or experienced pain at some point in their life, and everyone has taken some kind of drug to help ease their symptoms. My students have also been hurt or sick and taken medication. I envision that they will be extremely interested in understanding the basics of how drugs work in their bodies.
In my school district and around the world, there is a push to prepare students for the 21 st century. There has also been a movement to add more relevance and rigor to the curriculum. The original Bloom's Taxonomy was created in 1956, by a group of educators chaired by Benjamin Bloom. The taxonomy is a classification system of the thinking skills important to learning. A "new" Bloom's Taxonomy published in 2001 is designed to achieve relevance for 21 st century learners. By striving for the top of the pyramid, teachers will push their students to use higher–level thinking skills resulting in more relevance and rigor. 1
The six new categories of Bloom's Taxonomy beginning at the lowest level are: Remembering, Understanding, Applying, Analyzing, Evaluating and Creating. My curriculum unit utilizes every category, but the crux of the unit focuses on the top four categories.
The unit also makes a natural connection between math and science. I have known for a long time that it is wise pedagogically to take advantage when such opportunities arise, and to create such opportunities when possible. Using an integrated approach to teach math and science increases achievement in both disciplines. 2 When I have integrated math and science in the past my students are engaged and excited. Research indicates that this is not an accident, and in fact an integrated math and science unit lends itself to motivating students. 3
However, another reason to teach math and science in an intertwined manner is because the reality of teaching today is that teachers need to utilize every minute of the school day in the most productive and efficient way. By incorporating many skills into a lesson or unit, the class accomplishes much more in a shorter amount of time, thus freeing up additional time for learning other skills.
Background
The background information is broad and far reaching. How drugs work is high level content. The subject combines math and biology and chemistry. I have carefully selected concepts that will provide my students with a basic understanding of how drugs enter our body, how drugs are designed, and some basic principles to give my students an overview of science skills that play a part in the process.
Drug Delivery
Some substances can enter the body by passing through the skin. The students need to understand that the skin is a barrier designed to protect us. In order for medicine or any substance to penetrate the skin, there must either be a break in the skin or the substance has to be absorbed through the skin. Only a few drugs are capable of penetrating through intact skin. For those that do penetrate, transdermal patches or topical creams can be used to administer them. For others, they must be administered by injections (intramuscular, subcutaneous, intravenous) through the skin.
Various drugs are also administered to a patient through the mucous membranes such as the eyes, mouth, and ears to name a few. Oral administration, inhalation, and sublingual are methods in which medication or drugs are administered via the mucous membranes. Openings and cavities of the body are lined with mucous membranes, which are also exposed to the outside world, and have a protective barrier, so that not all agents can penetrate these barriers successfully either.
The chemical properties of drugs also affect how they are absorbed and distributed through the human body. Two important properties in this regard are molecular weight or size and whether the molecules are lipid or water soluble. The digestive system absorbs food and drug molecules based on these criteria. Small molecules usually diffuse more easily than larger molecules. Small molecules are able to pass through the wall of the small intestines to enter the bloodstream. Proteins, fats, and carbohydrates are larger in size, but the process of digestion breaks them into smaller pieces. However, some larger molecules that are not absorbed travel through the digestive tract unchanged.
Solubility also influences absorption and distribution. Because they do not move easily through cell membranes, water soluble drugs enter the blood and the interstitial fluid that is in between cells. Lipid soluble molecules that make up lipophilic drugs are fat–soluble. They do not dissolve well in water. Thus, since the human body, and particularly our blood is mostly water, this class of drugs does not get dispersed easily throughout the bloodstream. While the lack of water solubility is limiting, it does allow them to serve a different purpose. Fat soluble molecules penetrate easily through membranes. Thus lipophilic drugs can penetrate the skin and the capillaries in the brain. The molecular structure of a drug not only affects solubility, but also influences how it is designed and administered.
There are advantages and disadvantages of the various techniques of administering drugs. Bioavailability is an important concept to understand and plays an important role in determining the pros and cons of different types of drug administration. Bioavailability is the fraction of a drug that becomes available for circulation when delivered to the body (Saltzman 2012). For example, some drugs have 100% bioavailability in a given route of administration, but others do not. Some drugs metabolize, change or penetrate poorly and therefore only a portion of the drug enters into circulation. Below are brief descriptions of some of the many ways drugs enter our bodies and an overview of the advantages and disadvantages of each.
Intravenous injection
An intravenous injection, commonly referred to as an IV, uses a needle to inject a medication into a person's vein. Because the IV needle is inserted into a vein, the injected drug molecules enter the blood circulation. An advantage of an IV is that it has 100% bioavailability: the entire drug dose gets circulated through the bloodstream. Intravenous injections also have a high accessibility to tissues throughout the body because it takes only three beats of the heart to distribute the drug throughout the human body. Also an IV allows for control. If the physician delivers the injection, then the physician knows that the patient has received an exact dose of medication. A few disadvantages are that an injection is painful and requires a health care worker to administer it. Anytime a needle is used to provide an injection, there is always a risk of infection. There is also the risk of an allergic reaction or an overdose.
Intravenous infusion
An intravenous infusion uses a needle, but is hooked up to a bag and is called a drip. As with an IV injection, the bioavailability is also 100% and an IV infusion offers constant control over the amount of drug in the bloodstream. The disadvantages are the risk of infection and that an IV infusion requires hospitalization.
Subcutaneous injection
A subcutaneous injection is an injection that is given into the cutis, which is a deeper layer of the skin. The advantage is that the bioavailability is often high. A subcutaneous injection also allows the drug to avoid the digestive process and is absorbed slowly. Once again, a disadvantage is pain. Because the injection goes into the skin, and not into a vein, it is not as dangerous as an IV injection and many people can be taught to give SC injections to themselves.
Intramuscular injection
An injection that is administered directly into a muscle is an intramuscular injection. Insulin and vaccines are often delivered in this way. The positive aspects of this delivery method are that the drug avoids digestion, is absorbed more quickly than a subcutaneous injection, and provides control or certainty that the drug is delivered. An intramuscular injection is absorbed faster than the subcutaneous injection because there are more blood vessels in the muscles. The disadvantages are that the injection causes discomfort, may be distributed too slowly, may cause some painful side effects, and are usually not self–administered. Since the injection goes into the muscle and not into a vein, it is not as dangerous as an IV injection and can be self–administered if necessary.
Oral
Taking drugs orally is convenient and common in our society. Advantages of this method include self–administration and convenience. Disadvantages are that the drug begins to break down before it is absorbed and some drugs have limited bioavailability, because they do not readily penetrate the intestinal mucous membrane, which is required for absorption into the blood stream. With oral medications, if the bioavailability is low, then the dose can be adjusted (to make up for the drug that is lost or not absorbed) or another method of administration may be necessary. Since the medication is self–administered, there is less control both of the amount that is bioavailable and the compliance of individual. The medication may not be taken or it may be taken improperly.
Inhalation
Some medication is inhaled, particularly asthma medication, which is quite prevalent among school children. Inhalers provide for rapid delivery to the bloodstream. Also the structure of the lungs, which have a surface area roughly the size of a tennis court, allows for very efficient absorption. The disadvantage of inhaled medication is the potential for some drugs to damage the lungs.
Sublingual and Buccal
Sublingual medications are self–administered by holding them under the tongue. Buccal medications are similar to sublingual, but they are placed between the gum and the cheek. Both sublingual and buccal medications avoid the first pass in the liver. It is during the first pass where drugs are metabolized or changed. For some drugs the first pass greatly reduces the concentration that reaches circulation. A disadvantage is that sublingual administration is limited to lipophilic drugs, which are the only ones readily absorbed through the mucosal tissues. Because the surface area for absorption is so small compare to the lung or the intestine – the drugs must also be very potent. Nitroglycerin, which opens up blood vessels, is used to treat angina and is an example of a lipophilic, sublingual drug.
Ophthalmic
Drugs designed to treat the eyes belong to this group. Advantages of ophthalmic drugs include localization and self–administration. Disadvantages are the frequent dosing and that they may cause discomfort.
Topical
Topical medications are creams or ointments applied directly to the skin or other body surface. They are self–administered and used to treat a very localized problem such as a rash. Their use is limited to the area in which they are applied directly.
Transdermal
The transdermal patch is an adhesive pad that adheres to the skin and controls the dose of medication delivered through the skin to the bloodstream. The patch is self–administered. Transdermal systems are designed to provide a constant dose of drug over a period of many days. Because the drugs must be able to penetrate the skin, the patch is limited to lipophilic drugs. One limitation of the transdermal patch is that it can cause irritation to the skin.
The Human Body
Cells
Cells are the most basic unit of life, and the basic functional unit of all living things. The human body is made up of trillions of cells. While there are distinct differences there are many commonalities that all cells share. In humans, there are over 200 different types of cells such as nerve cells, blood cells, and muscle cells. A basic introduction to an animal cell and its parts will be shared with the students. Cells play an important role in how drugs work.
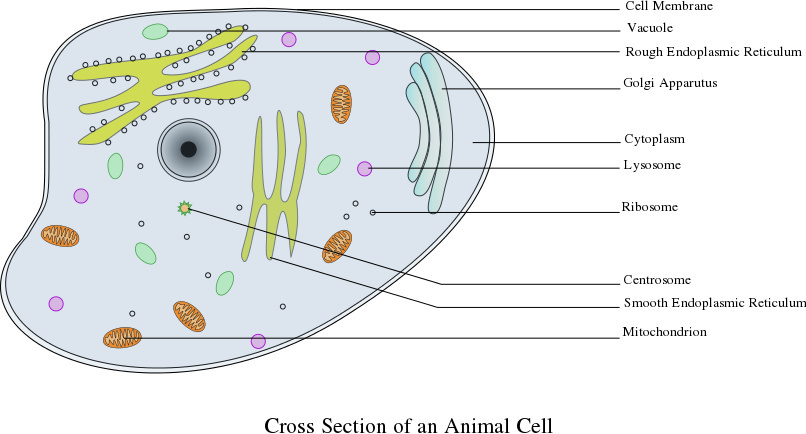
Figure 1
Tissues and organs
Cells of the same type are organized together and form tissues. Tissues are groups of similar cells that work together to perform a specialized function. The four main types of tissues found in the body are epithelial, connective, muscle and nerve. Two or more types of tissues that work together to perform a common function are called an organ. The liver, kidneys, and heart are examples of organs.
Organ systems
When two or more organs join forces to perform a function an organ system is formed. The circulatory, digestive, and nervous systems are examples. In the human body, there are many systems that are involved in enabling drugs to work. The immune system, the nervous system, the muscular system, the digestive system, the excretory system, the urinary system and the circulatory system are important. The focus of this unit is an overview of how drugs enter, disperse, metabolize, and exit the human body. So keeping my audience, fourth graders, in mind, the unit will explain briefly the role of the circulatory, digestive, and excretory systems.
The Circulatory System
The circulatory system is made up of the heart, blood vessels, and blood. The circulatory system is responsible for transporting nutrients, hormones, gases, and waste products throughout the body. In the process the circulatory system also distributes drugs that are present. So for most drugs when the molecules enter the body they eventually get to the bloodstream. Once in the bloodstream, the drug circulates throughout the body where it is available to enter all of the tissues and organs that are served by the circulatory system. The drug hooks up to a receptor site either in the brain or somewhere else. The drugs slowly get back to the bloodstream and eventually end up in the liver where excretion takes place.
The Digestive System
The digestive system is comprised of the mouth, esophagus, stomach, liver, small intestines, large intestines, rectum and anus. The digestive system is in charge of breaking down food into the nutrients that can be absorbed through the intestinal wall into the body: the nutrients allow the body to obtain energy and grow. The digestive system is activated with the smell of food. Imagine smelling homemade chocolate chip cookies baking in the oven. Before the cookies are removed from the oven you may find yourself salivating. Then, the cookies have to cool before they can even touch your mouth. In this scenario, the nervous system sends a signal to the brain, which sends a signal to the salivary glands. The glands salivate, and your mouth is watering. When the cookie finally reaches your mouth, extra juices are there to begin the digestive process. As you bite into the cookie, the carbohydrates in it get moist and mushy making it easier to swallow. The enzyme, amylase, which is present in saliva, begins to break down the carbohydrates in the cookie. The first bite is swallowed and you take another bite until the whole cookie is swallowed. Proteins, however, are different. Foods that contain proteins are chewed into smaller pieces in the mouth, but the proteins do not undergo a chemical change until further down the digestive tract.
The cookie proceeds down through the pharynx and esophagus. Involuntary muscle contractions in the esophagus, called peristalsis, push the cookie toward the stomach. A sphincter muscle at the end of the esophagus opens to allow food to pass into the stomach. Within the stomach there are glands that secrete acid, enzymes, and mucous: the acids and enzymes continue the process of molecular breakdown. The stomach muscles "knead" the mixture by contracting every 20 seconds resulting in what is called "chyme". The chyme is pushed into the duodenum, or the beginning of the small intestine. It is in the duodenum where the extraction of nutrients begins. The small intestines absorb water and nutrients. The nutrients that are absorbed pass through the intestinal wall and into the blood vessels for transport throughout the body. The food continues to break down from the action of bile, which is sent into the intestine from the liver, and enzymes, which are sent in from the pancreas. Once again, peristalsis in the small intestine serves to move the food along and to mix these juices together. The bolus of food moves from the duodenum to the jejunum to the ileum, and finally to the large intestines. The indigestible parts of food – i.e. those parts that were not absorbed in the small intestine – move to the large intestines where additional fluid is absorbed to produce waste. Once again involuntary muscle contractions force the material to move along the large intestine. Waste moves about 1 cm per hour. It can take days for the waste to move through the large intestines. Eventually the waste reaches the rectum where it exits the body.
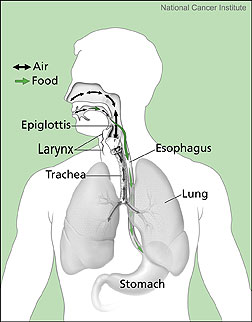
Figure 2
The Excretory System
The excretory system is comprised of the kidney, liver, lungs, and skin. The excretory system regulates the chemical make–up of fluids in the human body. The excretory system simply collects water and filters body fluids. This system removes metabolic waste and maintains a balance between the amount of salt, water and nutrients in the body. In simple terms, the excretory system metabolizes protein into amino acids. Many of those amino acids are reused in the building of other proteins, but some are further metabolized. A byproduct from the metabolism of amino acids is amino groups (NH 2), which combines with another hydrogen to form NH 3 , or ammonia, which is present in the body at low concentrations, but if the concentration builds, it becomes toxic. Some of the ammonia gets converted into another nitrogen–containing compound called urea. The body can handle higher levels of urea than ammonia. Urine, the fluid produced by our kidneys, contains concentrated urea. The production of concentrated urine is one way the excretory system is able to control the balance of water and wastes found in bodily fluids. This balance is called homeostasis. Drugs are excreted not only through the urine, but also through the lungs, bile, sweat, hair, breast milk, and tears. Urine, however, is the fluid where drugs – or the breakdown products of drugs – are most commonly found. 4
Drugs on the move
Once drugs enter the body, the various systems of the human body do their work to facilitate the process. In simplest terms a drug goes through four steps: absorption, distribution, metabolism and excretion. The method of drug delivery as well as the size and chemical structure of the drug impact the length of the process.
Absorption
The basic principle of diffusion is often involved in drug absorption. In diffusion, molecules move from an area of high concentration to an area of low concentration. Drugs molecules are absorbed into cells and tissues by crossing membranes. There are several different processes that molecules can use to cross these membranes. Passive diffusion or passive transport is one process used by many drug molecules. Passive transport is diffusion across a membrane. Drug molecules are either water soluble or fat soluble. Passive diffusion can occur with fat soluble molecules or with water soluble molecules. Fat soluble drugs are able to cross cell membranes more easily than water soluble molecules. Molecules that are fat soluble can easily pass through the wall of the small intestines if they are small. 5 Due to the ability to cross cell membranes and the blood–brain membrane, fat soluble drugs are absorbed more quickly than water soluble drugs.
Distribution
Once the drug molecules are absorbed, they enter the bloodstream. Then within one minute the heart circulates the entire supply of blood throughout the body. Thus, the heart pumps the drug molecule to all parts of the body. Drug distribution is the movement of a drug to various tissues as well as the proportions of drug in the tissue. Most drugs do not spread evenly throughout the tissues in the body. 6 The brain receives 16% of all the blood that is circulating. However, only certain nutrients, oxygen and fat soluble drug molecules can enter the brain. Only fat soluble molecules can cross the blood–brain barrier, which is the cellular barrier that surrounds the brain, whereas water soluble molecules cannot. Organs with high blood flow such as the brain, heart and liver are the first to accumulate drugs. The connective tissues and the organs that do not receive high blood flow are the last to accumulate drugs.
Metabolism
Metabolism can be defined as the chemical reactions that take place in living organisms. Digestion is one example of metabolism in living cells. Digestion is a process that takes place to help an organism live. As seen in the earlier example with the chocolate chip cookie, enzymes play a role in breaking down molecules. Digestion breaks down and changes molecules, separating the nutrients from the waste products. Drugs are metabolized also, but they are often more complex and difficult to break down than food. Some drugs are excreted intact, but most drugs that are in the blood and tissues have to be inactivated prior to being excreted from the body. During the metabolism, the structure of the drug molecule is often altered, thus creating a chemically related substance that can be more easily excreted from the body.
Excretion
The liver uses enzymes to break down the drug. During this process the drug is either broken into smaller pieces or altered, thus forming a drug metabolite. Once the liver changes the drug into smaller particles the kidney becomes involved. For example, the liver alters a fat soluble drug into a water soluble metabolite that can be excreted by the kidneys. The kidneys filter the blood for metabolites and waste, which are collected in the urine. The urine is passed to the bladder. Eventually the drug or metabolite is flushed from the body. Enzymes are not only used in the digestion and metabolism of drugs, but also in understanding how drugs work.
Enzymes
Enzymes are proteins that act as catalysts and speed up chemical reactions. Without enzymes, it would take 50 years for a meal to break down into small pieces that could be absorbed into the bloodstream. Enzymes like all proteins, are made up of a long string of amino acids. An enzyme protein may have between 100 and 1,000 amino acids connected together in a very specific order. And there are 50,000 to 100,000 different types of enzymes in our body. Some float around in the cytoplasm of cells, some are released into the water filled spaces between cells, and some circulate in the blood.

Figure 3
At first glance, enzymes appear to have the same shape, but at a closer view this is not true. The shape of the enzyme is important to how it works. Each enzyme has an active site. There is a cleft at the active site. A substrate can fit tightly into the active site. Once a substrate connects with the enzyme, a weak chemical bond forms, and the enzymes can get to work. After the chemical action is finished, the enzyme emerges unchanged and finds another substrate to which it connects. In addition to substrates, there are also inhibitors that have different shapes and can fit into the cleft of an enzyme. If the inhibitor fills in the space instead of the substrate, as its name suggests, it can prevent the substrate from entering the active site, and can inhibit the action. Most drugs work by interfering with an enzyme or a receptor. A receptor works in a similar way, but receptors are found on the cell membrane, the cytoplasm, or around the nucleus of a cell.
Scientific Investigation
The background information provided for this unit is designed to provide a framework for a series of scientific investigations that go beyond the typical "elementary school experiment." The idea is for students to explore scientific principles of cell membranes, solubility, diffusion, and equilibrium. The students will conduct various experiments and determine different ways to get an Alka–Seltzer tablet through a stocking membrane. The students will also explore how particle size affects the particles permeating the membrane and the time required for diffusion. Then the class will discover the layers that make up Advil or Tylenol. The class will observe a slide of a cross section of an over the counter pain reliever under a microscope. Further, students will design a drug using candy necklaces and homemade lollipops: they will need to perform a prolonged experiment and think like a scientist. In this experiment the students will dip beads from the candy necklace into the lollipop mix (see appendix B) to add a layer of coating. Then the students will determine the time it takes for the coating to dissolve in water. The students will manipulate the amount of lollipop coating by dipping the candy necklace bead numerous times to make the coating thicker. The idea is to encourage the students to use scientific inquiry skills. The students will not simply collect data to answer a few questions. Instead they will collect and analyze their data to drive their questions and reformulate their hypotheses. The students will work together to design a "drug" that will dissolve in a certain amount of time, for example 15 minutes. This type of scientific investigation not only involves the higher levels of Bloom's Taxonomy, but also provides relevance and rigor as math and science are integrated in a meaningful way.
In order to guide students through the drug design, students and teachers need to be familiar with the process of scientific investigation. The independent variable or the manipulated variable is the one part of the experiment that is deliberately changed. For example, in this experiment, the independent variable would be the amount of lollipop coating applied to the candy necklace beads. The amount of lollipop coating can be increased by applying a layer, allowing it to harden, then applying another layer, etc. thus mimicking the way many drugs are layered by design. The independent variable or the layering of the lollipop coating is determined and controlled by the experimenter.
The controls or constants in the experiment are all of the parts of the experiment that stay the same. The candy necklace beads, the location of the experiment, the temperature of the melted lifesaver, the volume and shape of the beaker that holds the melted lifesaver, and the thread used to dip the candy necklace beads into the beaker would be the constants. It is important for the students to understand that everything except for one thing, the independent variable, has to be held constant in the experiment. If more than one independent variable is changed, it is not clear what the one factor was that impacted or changed the results.
The dependent or responding variable is the responding "thing" that happens as a result of the changes in the independent variable. For example in this experiment, the responding variable is the amount of time it takes for the lollipop coating to dissolve off of the candy necklace beads into the water. The responding variable is not something that the experimenter can control. It is what happens as a result of the change in the independent variable.
The students will collect their data, analyze their data, and graph their data. The appropriate graph for this type of experiment is a line graph since the experiment is looking at change over time.
Strategies
The two main strategies that will be used throughout this unit are scientific investigation and integrating math and science in a meaningful, natural way. The scientific investigation is designed to get the students engaged by "doing science." The students will begin by learning the process skills necessary to carry out an investigation. The investigation, or experiments will start out with teacher guidance. The experiments will be scaffolded, so the students develop skills prior to the investigation and then self direct their investigation and hypothesis for creating a "drug" that will dissolve in a set amount of time.
The unit also includes hands–on kinesthetic learning experiences where the students are moving about the room to learn about enzymes. Additionally, the students will incorporate some written explanations as well as oral language skills as they work together in small groups.
The math objectives will be taught through application. Graphing, analyzing data, using fractions and measuring elapsed time are skills that will be developed in the natural context of the unit.
Activities
Overview of Daily Schedule
Day 1
The students will work in cooperative groups to brainstorm different ways that medication enters the human body. The results will be compiled into group posters.
Day 2
The teacher will provide the students with a handout of the different ways drugs are delivered. The students will record notes about the pros and cons for each type of delivery.
Day 3
The students will work in cooperative groups to brainstorm different ways an Alka Seltzer tablet can penetrate a stocking membrane, without removing the membrane.
Day 4
Students will be provided with several cups with stocking membranes. They will also be given supplies based on their brainstorm list. Then the students will test out their methods to see if they can successfully pass through the membrane. The small groups will share their results with the class.
Day 5
A brief overview of cells, tissues, and organs will be delivered. The students will make a diagram of an animal cell.
Day 6, 7 & 8
The students will learn about the circulatory, digestive, and excretory system. The students will complete coloring activities using pages from a human anatomy coloring book.
Days 9–11(activity 1)
The class will have a mini lesson on diffusion and equilibrium. Then the students will conduct experiments to determine how the temperature and particle size affect diffusion. First, the students will alter and record the temperature of the water to determine the effect it has on the time it takes for food coloring to diffuse and reach equilibrium. Next, the students will experiment with the time it takes for an Alka Seltzer tablet to diffuse and reach equilibrium if it is whole, cut into fourths, or ground into a powder. The students will record the results. The last set of experiments will have the students assist the teacher to make sodium alginate beads. There are websites with videos that demonstrate this process (see resources appendix). Then the alginate polymers will be used to conduct a third experiment where the students will observe the diffusion process. If food coloring is added to the alginate when making the polymers, then the coloring will diffuse out of the polymers when placed in water. The students can observe this process until equilibrium is reached. However, if poster paint is added to the alginate when making polymers, then the molecules are too large and will not diffuse out into the water. Thus, providing a model of how some molecules can move through membranes and others cannot. The experiments will conclude with the teacher informing the students of the processes of absorption and distribution and the role diffusion and equilibrium play.
Day 12
The teacher will introduce information about metabolism and excretion.
Day 13 (activity 2)
The teacher will teach the students background information about enzymes and how the active site binds to a substrate or an inhibitor. Then the class will partake in a game to demonstrate how the active site works in the case of aspirin. In preparation for the game, the teacher will purchase several blank (all white) jigsaw puzzles. The teacher will color the outer edge pieces of both puzzles yellow. Then color the adjacent interior row of one puzzle red and the adjacent interior row of the second puzzle blue. To play the game, the teacher will give each student either a yellow, red, or blue piece of the puzzle, making sure that every red or blue piece has a yellow with which to interlock. Each yellow piece in the game should have a red and a blue match. When the game begins the students have to find their match. Since a red and a blue piece match each yellow piece, some students will be unable to find their match or get into the "active site". Once all yellow pieces have a match, the teacher will pull a color, red or blue, out of a hat. The color that is called is the "inhibitor" that prevented the pain. All matches made with the inhibitor get a point. For example, if blue was the inhibitor, then all of the students that are involved in the blue and yellow matches earns a point or a token. The pieces would be collected and passed out again. The game can proceed for a given amount of time or until a certain number of points or tokens are attained. At the conclusion of the game the teacher should review the concept of active site, substrate and inhibitor.
Day 14 (activity 3 begins)
The class will string a bead from a candy necklace through a piece of thread. Then the students will dip the bead into the beaker of lollipop mix. Each group will make three beads for the designated number of lollipop dips. The students will make three beads with one, two, three, and four coatings. The coated beads will be placed on labeled parchment paper to indicate the amount of dips. The students will also determine the mass in grams of each bead and record the data.
Day 15–18 (activity 3 continues)
The experiments will begin to determine the elapsed time for the lollipop coating to dissolve. Each group will conduct three trials and record the results. On day 15 the students will conduct the experiment for the candy beads that were dipped once. Following the experiment, the students will hypothesize the amount of time it will take for the beads with two coats to dissolve. On day 16 the students will conduct this experiment and record their results. After the experiment, students will again make hypotheses about the time it will take for the beads with three coats to dissolve. This process will continue on Day 17 and Day 18 for the beads with three and four coats respectively. The students will share the results and the teacher will help the students to analyze the relationships particularly between one and two coats and two and four coats to determine if double coating results in double the dissolve time.
Day 19–21(activity 3 concludes)
The students will work in the same cooperative groups. On day 19, they will analyze their data and develop a plan to create a "drug" that will dissolve in 15 minutes. The group will write a paragraph to explain their rationale. On day 20, they will once again create their beads. Each group will apply the lollipop coating to three beads according to their plan. On day 21, the big competition will take place to determine which group has the smallest margin of error based on elapsed time.
Day 22
The teacher will provide each group of students with a series of interview questions about what they learned. Each group will divide the questions up amongst the group and prepare their answers.
Day 23
The students will be interviewed and their responses will be videotaped.
Appendix A: Teacher Resources
http://gelfand.web.cmu.edu/scimodules/2._Gel_Beads_and_Worms.html
This website shows alginate polymers made with food coloring and paint. It also provides one vendor for ordering alginate.
http://www.youtube.com/watch?v=u1IMibjMufc
A great video to provide an overview of making alginate polymers. The video is less than two minutes long, and is very informative.
Appendix B
Recipe – lollipops
1 cup sugar
½ cup light corn syrup
¼ cup water
1 teaspoon cream of tartar
food coloring (if desired)
flavoring (if desired)
Combine and stir sugar, corn syrup, water, and cream of tartar.
Bring to a boil on medium–high heat while stirring.
Insert candy thermometer and let boil without stirring until the temperature reaches 295F.
Remove and let sit until no longer boiling.
Stir in food coloring.
I heated a few smaller oven safe containers in the oven at 250F. I poured the mixture into smaller oven safe containers to keep it warm throughout the process.
Appendix C: Implementing District Standards
Science
4.1 The student will demonstrate an understanding of scientific reasoning, logic, and the nature of science by planning and conducting investigations in which
a) distinctions are made among observations, conclusions, inferences, and predictions;
b) objects or events are classified and arranged according to characteristics or properties;
c) appropriate instruments are selected and used to measure length, mass, volume, and temperature in metric units;
d) appropriate instruments are selected and used to measure elapsed time;
e) predictions and inferences are made, and conclusions are drawn based on data from a variety of sources;
f) independent and dependent variables are identified;
g) constants in an experimental situation are identified;
h) hypotheses are developed as cause and effect relationships;
i) data are collected, recorded, analyzed, and displayed using bar and basic line graphs;
j) numerical data that are contradictory or unusual in experimental results are recognized;
k) data are communicated with simple graphs, pictures, written statements, and numbers;
l) models are constructed to clarify explanations, demonstrate relationships, and solve needs; and
m) current applications are used to reinforce science concepts.
4.5 The student will investigate and understand how plants and animals, including
humans, in an ecosystem interact with one another and with the nonliving components in
the ecosystem. Key concepts include
a)plant and animal adaptations;
Math
4.6 The student will
a) estimate and measure weight/mass and describe the results in U.S. Customary and
metric units as appropriate;
4.9 The student will determine elapsed time in hours and minutes within a 12–hour
period.
4.14 The student will collect, organize, display, and interpret data from a variety of
graphs.
Endnotes
- Forehand, Mary. "Bloom's Taxonomy – Emerging Perspectives on Learning, Teaching and Technology." Projects Server Introduction. http://projects.coe.uga.edu/epltt/index.php?title=Bloom%27s_Taxonomy (accessed July 16, 2012).
- As quoted in Furner, Joseph, and David Kumar. "The Mathematics and Science Integration Argument: A Stand for Teacher Education." Eurasia Journal of Mathematics, Science & Technology Education 3, no. 3 (2007): 186. www.ejmste.com/v3n3/EJMSTE_v3n3_Furner&Kumar.pdf (accessed July 12, 2012).
- IBID.,186.
- "BASIC PHARMACOLOGY." Dr. David Benjamin – Forensic Toxicologist, Pharmacology, Toxicology; Analysis of Blood, urine and hair drug tests; Cocaine/Narcotics issues; Vehicular homicide; OUI/DWI Defense, Breathalyzer Variability; Seminars. http://www.doctorbenjamin.com/pharm/pharm.htm (accessed July 16, 2012).
- Aldridge, Susan. Magic molecules: how drugs work. 2nd ed. New York: Cambridge University Press, 2008, 1.
- " Drug Distribution: Administration and Kinetics of Drugs: Merck Manual Home Edition ." THE MERCK MANUALS – Trusted Medical and Scientific Information. http://www.merckmanuals.com/home/drugs/administration_and_kinetics_of_drugs/drug_distribution.html (accessed June 29, 2012).
Annotated Bibliography
"Drug Distribution: Administration and Kinetics of Drugs: Merck Manual Home Edition." THE MERCK MANUALS – Trusted Medical and Scientific Information. http://www.merckmanuals.com/home/drugs/administration_and_kinetics_of_drugs/drug_distribution.html (accessed June 29, 2012). This website was very useful and reader friendly.
Aldridge, Susan. Magic molecules: how drugs work.. 2nd ed. New York: Cambridge University Press, 2008. A great overview about how drugs work. It contains high level information, but is written for a general audience.
Forehand, Mary. "Bloom's Taxonomy – Emerging Perspectives on Learning, Teaching and Technology." Projects Server Introduction. http://projects.coe.uga.edu/epltt/index.php?title=Bloom%27s_Taxonomy (accessed July 16, 2012). This site provided the latest Bloom's Taxonomy and background information related to creating an updated version.
Furner, Joseph, and David Kumar. "The Mathematics and Science Integration Argument: A Stand for Teacher Education." Eurasia Journal of Mathematics, Science & Technology Education 3, no. 3 (2007): 186. www.ejmste.com/v3n3/EJMSTE_v3n3_Furner&Kumar.pdf (accessed July 12, 2012). This is an academic research article outlining the reasons to integrate math and science.
Jeffries, Melissa. "HowStuffWorks "The Large and Small Intestines"." HowStuffWorks "Science". http://science.howstuffworks.com/environmental/life/human–biology/digestive–system2.htm (accessed July 16, 2012). A useful background about the digestive organs.
"BASIC PHARMACOLOGY." Dr. David Benjamin – Forensic Toxicologist, Pharmacology, Toxicology; Analysis of Blood, urine and hair drug tests; Cocaine/Narcotics issues; Vehicular homicide; OUI/DWI Defense, Breathalyzer Variability; Seminars. http://www.doctorbenjamin.com/pharm/pharm.htm (accessed July 1, 2012). This website provided accurate and thorough information. I consulted this article frequently to build my background knowledge.
Comments (0)
THANK YOU — your feedback is very important to us! Give Feedback
