- Login
- Home
- About the Initiative
-
Curricular Resources
- Topical Index of Curriculum Units
- View Topical Index of Curriculum Units
- Search Curricular Resources
- View Volumes of Curriculum Units from National Seminars
- Find Curriculum Units Written in Seminars Led by Yale Faculty
- Find Curriculum Units Written by Teachers in National Seminars
- Browse Curriculum Units Developed in Teachers Institutes
- On Common Ground
- Publications
- League of Institutes
- Video Programs
- Contact
Have a suggestion to improve this page?
To leave a general comment about our Web site, please click here
It Don't Come Easy: The Promises and Challenges of Biofuel Production
byRobert C. McDowellIntroduction
My students generally believe in climate change. They see it in the news, and they experience it in ever growing storms and weather events. They know it is bad, but they don't really understand the root causes. They have some knowledge that "air pollution" is the cause, but they don't have a grasp of the basics. My goal with this curriculum unit is to clear up some of the misconceptions, provide students with some possible solutions to our ever growing carbon problem, and help them understand that the choices we make as individuals and society will have profound effects on the future climate of Earth.
Kids know that cars and trucks run on "gas", and that the "gas" is somehow related to air pollution. They don't really know the origin of the fuel that they put into their automobiles, nor do they fully understand the effects that gasoline consumption has on the atmosphere. It has been well demonstrated that the use of traditional fossil fuels such as gasoline as motor fuels is having a profound effect on the climate. 1 The use of fossil fuels such as gasoline is a major contributor to carbon dioxide entering the atmosphere. Biofuels such as ethanol and butanol are increasingly being touted as a means of lowering our carbon emissions and increasing our fuel supply. By having my students explore some of the issues surrounding the production and use of biofuels, I hope to give them a very solid understanding on which they can base any future opinions or ideas.
There are a few key learnings from this curriculum unit that will leave my students well educated in the issues surrounding biofuels and their use as a motor fuel. First they will discover why biofuels such as ethanol hold so much promise in the campaign to lower our carbon emissions. My students will also explore why biofuels derived from sources such as corn are not as environmentally friendly as initially promised. 2 Some of the difficulties in producing ethanol directly from cellulosic sources will be explored, as well as comparisons of the different energy quantities, production methods, and benefits of ethanol and butanol. The students will come away with a good understanding of how biofuels can lower greenhouse gas emissions, how they are made, and also some of the drawbacks to the production and use of biofuels. Finally, students will be asked to incorporate what they have learned into the overall concepts of sustainability and environmental unity.
By introducing concepts in the sequence described above, I will be able to get students to see the "big picture" and make a connection to the entire curriculum of my AP Environmental Science class. They need to be able to tie concepts together and see the environment as a whole, and not as a collection of discrete problems. To enable my students to grasp the overall concept and controversy of biofuel production, I need to help them understand the environmental, cultural, and economic aspects that determine the sustainability of an issue.
By studying the issue of biofuels, students will see how environmental issues are tied to other issues such as economics and politics. They will hopefully better understand two of the unifying principles throughout environmental science. The first is the principle of sustainability. Students will need to understand that the choices that are made today will have a long lasting effect, and will need to be acceptable by society in order to be adopted. Anything we choose to do today should be sustainable for future generations and the environment they will occupy. I work with my students so they understand that no action or introduction is simple. Whatever motor fuel choices we make today need to be sustainable in the future, and need to address the problems associated with the use of fossil fuels as motor fuel.
The second important principle for my students to learn is that of Environmental Unity. This is the concept that no action or occurrence happens in isolation in the environment. An action taken in one area can have very devastating consequences in an area of the environment very far away. For example, the increased use of corn as a basis for ethanol production has led to an increased use of nitrogen fertilizers to grow the corn. This fertilizer has washed off of farm fields in the Midwest, and ended up in the Gulf of Mexico where it has caused a great deal of harm to aquatic organisms. 2 The farmers in the mid west do not intend to harm the Gulf organisms, but the effect is real, and is a good example of Environmental Unity.
I teach at Newark High School in Newark, Delaware. My school is fairly large, with a student population of approximately 1700 students. We are a Title 1 school, with over 50% of our students eligible for free or reduced lunches. I have been teaching at Newark High since 2000, and I have been teaching AP Environmental Science since 2004. My typical science class has about 25-30 students, of varying backgrounds and academic abilities. My AP classes are more homogeneous than the lower level Biology classes that I also teach, but there is still a fair degree of academic variability in those classes.
The intended audience for this unit is 11 th and 12 th grade students enrolled in my AP Environmental Science class. The various activities incorporated into this unit of curriculum are designed to engage students who will be involved in these types of activities in a college level environmental science class, or as environmental scientists in the field. The debate over biofuels will intensify in the future, and hopefully this curriculum will allow my students to be well prepared to discuss any of the ramifications in a well grounded manner. In addition, several key Science Standards for the State of Delaware and the College Board are addressed (see Appendix 1).
My classes are arranged in an A/B block schedule. I see the students for 90 minutes, every other day. The activities and lessons contained in this curriculum are designed for the 90 minute block. However, they should be easily modified to fit into any school schedule with slight modifications.
I intend to teach these units concurrently, and not break them up over time. This curriculum will be taught as a discrete set of lessons within my overall Energy Unit. It should take me approximately two weeks on Block scheduling to cover all of the material in this curriculum unit.
The activities in this curriculum unit will mimic actual tasks that a research scientist or environmental lab technician would do as part of their job. By having the students perform actual hands-on activities I hope to engage them in a higher level of interest, and thereby help them incorporate the ideas within this curriculum in their greater environmental learning. This hands-on method also makes the information that is collected from many on-line sources more authentic since students will be collecting some of their own data, and drawing their own conclusions about the merits of biofuels.
Students will be exposed to ample scientific research and literature so they can see the current and historic impacts of biofuel usage. I will provide them with sets of printed articles to read as homework in order to provide them with adequate background on the topic before we begin each series of activities. Students will evaluate findings of relationships between corn ethanol and the environment. They will be expected to evaluate scenarios to determine the potential for environmental impacts, and to use critical thinking to come to conclusions about which biofuels and production practices are best suited for sustainable development.
Background
The world faces many new energy related challenges as we move further into the 21 st century. Among these challenges are the declines in easily obtained fossil fuel reserves, and more importantly, the carbon emissions that using fossil fuels create. The burning of fossil fuels produces around 21.3 billion tons of carbon dioxide per year. It is estimated that natural processes absorb about half of that amount, so there is a net increase of 10.65 billion tons of atmospheric carbon dioxide per year. 1 Carbon dioxide is one major greenhouse gases that contribute to global warming and climate fluctuation.
One possible solution that would ameliorate some of both of these crucial problems would be to switch at least some of our motor fuels from traditional fossil fuels such as oil and gasoline to so-called biofuels. The ability of plants to capture CO 2 from the atmosphere and convert it into harvestable biomass is the cornerstone of biofuel production. Biofuels are a possible answer to the carbon pollution of traditional fossil fuels because they are considered carbon neutral. Such fuels are potentially because they do not result in a net increase in atmospheric .This means that the carbon that is released by burning the fuel is eventually taken up again and converted into the biomass used to produce new fuel. The carbon does not permanently remain in the atmosphere, and therefore biofuels are considered a better alternative to fossil fuels.
Biofuels consist of a fairly wide variety of fuels that are converted from biomass. Probably the most widespread and highly visible biofuel is ethanol. Ethanol currently comprises at least 10% of what we commonly use as fuel in a gasoline powered vehicle. Most automobiles in the United States run on blends of up to 10% ethanol (E10). Most gas pumps have some visible indication on the pump that the gasoline being dispensed has at least 10% ethanol mixed with the gasoline. Pumps that do not have ethanol mixed with the gasoline also have very visible stickers indicating that they do not dispense any ethanol along with the gasoline. Flexible-fuel automobiles and trucks use gasoline/ethanol blends ranging from pure gasoline up to 85% ethanol (E85). There are already approximately 11 million E85-capable vehicles on U.S roads. 3 The ethanol is used as a fuel extender, and as an additive to increase the octane rating of gasoline fuel. In the United States, most of the ethanol used for fuel is derived from corn which is grown specifically for use in ethanol production. 4
Some nations such as Brazil produce ethanol by the fermentation of sugars from sugar cane. Research in Brazil has centered on the introduction of better producing varieties of sugar cane, and creation of policies that support sustainable land management. 5 In the United States, most ethanol production has involved the use of corn as a feedstock. Ethanol production in the U.S. topped 4 billion gallons in 2005 and consumed 1.4 billion bushels of corn, valued at $2.9 billion. This represents the third largest demand for U.S. corn after animal feed and export markets. With additional construction of ethanol plants and increasing ethanol demand, ethanol fuel production exceeded 7.5 billion gallons before the year 2012 target set forth in the Energy Policy Act of 2005. 6
Corn and sugar based ethanols are considered "first generation" biofuels. Due to increasing global demand, biofuel production is expected to increase significantly, mainly due to the expansion of first-generation biofuels in developing nations such as Brazil, China, and the United States. 7
One response of corn growers to the need for more and more corn for ethanol has been to intensify their farming practices. One of the largest practices is to adopt a "high yield" cropping system. High-yield cropping systems require fossil-fuel inputs to substitute human and animal labor and to maximize capture and conversion of solar radiation into crop biomass. Inputs to agricultural systems that require fossil fuel in their manufacturing process include fertilizer, seed, pesticides, and machinery. Fossil fuel also is required for application of fertilizers and pesticides, and for irrigation. Because fossil fuel combustion results in carbon dioxide emissions, any energy inputs raise the level of carbon dioxide pollution, and decrease the net benefits of corn ethanol even further. Another potential negative aspect of corn ethanol production is the potential for indirect land use change and associated carbon dioxide production from clearing of carbon-rich natural ecosystems for crop production. There has been a marked increase of land that is marginal being cultivated to ethanol corn production in the United States. 8
Generally speaking, first-generation biofuels consume much more water throughout their life cycle, when compared with other energy carriers. In fact, corn based biofuels exhibit much greater water footprints, sometimes by two or three orders of magnitude, than other energy carriers This is mainly a consequence of the high direct water use during feedstock production. Nevertheless, different biofuels have radically different water requirements, depending on the feedstock, the region where it is produced, and the production practices adopted. 9
Most guidance for calculation of the carbon footprint of biofuels, and most published carbon footprints, presume that biomass fuels are carbon neutral. However, it is recognized increasingly that this is incorrect: biomass fuels are not always carbon neutral. Indeed, they can in some cases be far more carbon positive than fossil fuels. The nature and the magnitude of overall biofuel carbon contributions depend on the biofuel feedstock, the mode of feedstock production, the agricultural practices adopted during feedstock production, the environmental and socioeconomic context of biofuel production, the stage of the biofuel's life-cycle, and the policies in place during biofuel production, use, and trade. 7
There are seven stages to a biofuels "life cycle". They are: 1) Site selection and preparation, 2) Feedstock growth and preparation, 3) Feedstock transportation, 4) Feedstock treatment and biofuel production, 5) Biofuel transportation, 6) Biofuel storage and dispensing, and 7) Biofuel combustion. 7 There are efficiencies to be gained in any of these steps, but my classes will focus on the feedstocks and the production of the different biofuels. We will also discuss the first and second stages as part of our Agriculture and Farming units, and thereby better tie the agricultural portion of the production of ethanol to the uses and actual production o the fuels themselves.
Because most of the ethanol produced in the United States is made from corn and the energy required by ethanol distilleries comes mostly from fired power plants, there has been considerable debate on the environmental benefits of corn-based biofuels in replacing traditional fossil fuels. Concerns relate to the large amount of land required for crops and its impact on , as well as issues regarding its real and also issues regarding water use and pollution due to the needs of the corn crop itself all the way to the final ethanol production. 10 The use of corn as a fuel source is controversial on several points, and attempts are being made to utilize feedstocks other than the starch in corn kernels for ethanol production. 11
One promising alternative feedstock for ethanol production is cellulose. 12 Cellulose is an with the ( 6 1 0 5) n, a polysaccharide consisting of a linear chains of Β(1→4) linked units. The length of individual cellulose chains varies greatly. Cellulose is the most abundant organic polymer on Earth, and is an important structural component of the primary of .
The linkage pattern of the glucose sub-units in cellulose is different than the glucose-glucose bonds in starches, glycogen and other carbohydrates. Unlike starch, cellulose is a straight chain polymer. The multiple hydroxyl side groups on the glucose from one chain form with oxygen atoms on an adjacent chain, holding the chains firmly together side-by-side and forming fibers with high tensile strength. NNN
Cellulose that comes from plants is usually found in a mixture with , , and other substances. The character and relative ratios of these other substances varies from plant species to plant species. The hemicelluloses and lignin serve as binding agents, holding the cellulose fibers in an even stronger matrix which ultimately gives plant their durability and strength.
Many physical properties of cellulose depend on its chain length and the number of hydrogen bonds between glucose units that make up a cellulose fiber. The degree of lignification of the individual cellulose fibers also determines to a great extent the overall strength of the cellulose structure being constructed. Cellulose from wood has typical chain lengths between 300 and 1700 units and are heavily lignified to neighboring cellulose units, while cotton and other plant fibers have chain lengths ranging from 800 to 10,000 units and have much less lignin within the fibers. 13
Cellulose is available in many forms, including agricultural waste and native grasses. One of the main proposed strategies for cellulosic biofuel production is widespread planting and harvesting of perennial grasses, such as switchgrass (Panicum virgatum L.) or miscanthus (Miscanthus X giganteus). One study suggested that net carbon dioxide savings relative to fossil fuels of greater than 200g of carbon dioxide /m 2/yr may be expected for biomass (switchgrass) conversion to ethanol. 14 Studies have been conducted and shown that the use of some agricultural wastes for ethanol production is also environmentally sustainable. 15
Production quantities of cellulosic ethanol have consistently failed to meet expectations, mostly because those production expectations were set based on amounts of cellulosic material available on fields rather than the ability of technology at the time to turn biomass into fuel. 12
One of the main problems with cellulosic ethanol production is that, unlike the starch sugars in grains, the complex polysaccharides in the cellulose of plant cell walls are locked within the lignin. 16 For advanced biofuels to be economically competitive, scientists must find inexpensive ways to release these polysaccharides from their lignin bindings and reduce them to fermentable sugars that can be synthesized into fuels. Studies are being conducted to determine how to optimize the use of agricultural waste to produce more accessible cellulose for ethanol production. 17
A key consideration when assessing biofuel sustainability is the extent to which a biofuel provides a net-energy gain when compared with the conventional fossil fuels it displaces. Two commonly used indicators are the energy return on investment (EROI) and the percentage fossil energy improvement. The EROI is the ratio of the total energy supplied by biofuel combustion (Eout) to the total energy used during biofuel production (Ein). Values of EROI greater than 1 imply net-energy gains. The percentage fossil energy improvement provides a measure of the amount of nonrenewable energy used during the life cycle of a biofuel. The EROI and percentage fossil energy improvement are usually measured as the carbon output through the complete life-cycle of the biofuel. 7
Biobutanol is another alcohol that can be derived from biomass, and has a greater energy content than ethanol. It is also considered a second generation biofuel. Because of its greater energy content per molecule, butanol been proposed as a better alternative fuel than either corn or cellulose-based ethanol. Butanol has more energy per unit volume than ethanol, and approaches gasoline in its energy content. 18
New discoveries could make the alternative fuel butanol more attractive to the biofuel industry by lowering production costs and eliminating some of the production bottlenecks. 19 Bacterial strains have been identified that can ferment biobutanol directly from cellulosic sources. 20,21 This would allow butanol to be produced in great quantities without some of the limitations of ethanol. Because of its higher energy content, butanol could potentially eclipse ethanol as the biofuel of the future. A switch from corn-based ethanol to either cellulosic ethanol or butanol would lower the environmental impact of biofuel production. 22
Implementation Strategies
Students will be exposed to ample scientific research and literature so they can see the current and historic impacts of biofuel usage. I will provide them with sets of printed articles to read as homework in order to provide them with adequate background on the topic before we begin the series of activities. I don't have a specific set of articles, as I continually update them to reflect the current state of research and technology. Students will evaluate findings of relationships between corn ethanol and the environment. Students will be expected to evaluate scenarios to determine the potential for environmental impacts, and to use critical thinking to come to conclusions about which biofuels and production practices are best suited for sustainable development. Classroom discussions and group activities will be a large part of the curriculum unit. I do present notes using my classroom projector, but the bulk of the learning in this unit will be group hands-on activities and classroom discussion.
Content Objectives
With the completion of this unit, students will be able to explain the role of fossil fuels in carbon pollution and climate change. They should be able to describe the characteristics of a biofuel and the relative benefits of biofuel use over fossil fuels as a motor fuel.
Students should be able to describe the costs, both economically and environmentally, of biofuels as well as the basic process for production of ethanol from corn. They should be able to relate the production of corn ethanol to some other environmental problems using the principle of Environmental Unity.
Students should be able to describe the basic process for production of ethanol from cellulosic sources, the benefits for the environment of cellulosic ethanol over corn ethanol, and the challenges of cellulosic ethanol production. They should also be able to describe some methods by which cellulosic ethanol production could be optimized.
Finally students will be able to describe the benefits, both economically and environmentally of butanol over ethanol as a potential biofuel of the future.
Curriculum Strategies
Students will be encouraged to think independently and make sense of the data discussed in class or observed in lab work. I have found that having students work on data collection and data analysis on their own makes them think harder, and put more effort into their understanding of a topic. I don't like to assign labs where students just fill in blanks and don't come to a good understanding of the topic. Also based on my prior experiences with labs, I will have students work in collaborative groups of three or four. I also do intend to use direct instruction, such as PowerPoint or chalkboard notes throughout the unit as a means of communicating larger ideas and concepts that are not amenable to lab studies. For example, I will need to go over the bonds in the various molecules, and explain how to calculate bond energies. This is because I do have students who will be in the middle of Chemistry in their 11 th grade year, and will not have seen bond energies calculated in Chemistry class yet. Videos as well as video clips and web based instruction will also be a big portion of the classes. I have videos that show farming techniques, and I will show these to illustrate the carbon inputs necessary for ethanol production.
Lab work will form a large component of the unit. Students will start by studying historical data about biofuels and their use as a motor fuel. This will familiarize them with and standardize their data collection techniques. Each collaborative group will ultimately present their own data and opinion paper on the benefits and challenges of various biofuel production and use.
Students will be placed into research groups that will research and present their research and lab work to the class. Each student will be given relevant articles about different aspects of biofuel production and use. Articles will also be posted to my web page for them to read.
Content related lectures and hands-on labs will help to generate curiosity and the interest of the students as the unit progresses. Having the students choose the format of their presentation will help heighten the interest of the students in the subject matter and may facilitate better questioning by the students.
Lesson 1: Carbon Content of Fuel Combustion
The first activity that the students will participate in is the mathematical calculation of the mass of carbon dioxide that is produced from a given amount of gasoline. I want them to understand exactly how much carbon dioxide pollution comes from the combustion of gasoline as a motor fuel. This is critical to engage them in understanding that the carbon from fossil fuels has been locked away from the atmosphere for millions of years, and that by combusting gasoline they are releasing carbon which has been sequestered in the Earth's crust. I want them to get an idea of just how much pollution they themselves create every day, in order to get them interested in the possible solutions they will be researching. Based on the Law of Conservation of Matter, which states that matter may be neither gained nor lost in a chemical reaction, students will calculate the amount of oxygen required to fully combust gasoline. They will also calculate the mass of Carbon Dioxide and water vapor that is created in the combustion process.
The essential learning from this activity is that the combustion of gasoline produces a large quantity of carbon dioxide as a product. The students will most likely be surprised at the amount of carbon dioxide that is produced from the combustion of gasoline.
Activity 1: Calculation of Carbon Dioxide Pollution from Gasoline Combustion
The actual activity is fairly straightforward. Students will receive a handout with a structural drawing of a hypothetical gasoline molecule (See Appendix 2). They will be informed that a 100% combustion efficiency is obtained, to simplify the calculations and make their calculations uniform.
Students will work in pairs or groups of three. They will be told that the products are carbon dioxide and water, and will first need to write an equation that shows the combustion of gasoline. The student groups will then have to balance the equations, and derive the number of moles of carbon dioxide that are produced per mole of gasoline combusted.
Activity 2: Calculation of the Carbon Dioxide Pollution Produced per Student, Efficiency Calculations
Now that students understand how much carbon dioxide is released from the combustion of gasoline, I will get them to think about fuel efficiency and how they can lower the amount of carbon dioxide that they produce as they drive.
I will keep the students in the same groups as they were in previously for this activity.
I will randomly assign a number that represents the miles per gallon average that their vehicle gets. I will tell them all that they drive an average of 10,000 miles in a year. They will then have to calculate the amount of carbon dioxide that they produce from their vehicle. Once they have calculated their carbon dioxide emissions, I will have them estimate the carbon dioxide emissions for the United States if one million people drove their type of automobile for the same number of miles each year.
I will ask them to come up with some variables that are probably not taken into consideration for this calculation. For example, not everyone drives 10,000 miles per year. The students will write their ideas in a sheet of white paper to be hung around the room as they discuss their answers.
Homework Assignment:
I will assign each student to research ways in which to increase the mileage of their assigned automobile. Methods will need to be realistic and easily achieved by the average motorist.
When the class reconvenes during the next block, I will have the students sit with their group. They will discuss the various ways that they have discovered to raise their automobiles mileage. The group will then prioritize what they feel are the ten best ways to increase gas mileage. Once all of the groups are ready, they will stand and present their findings to the class. The class will then prioritize a list of conservation tactics that they feel are feasible. I will take the class out to the parking lot and show them easy ways to increase their fuel mileage. For example, I will point out where a typical air filter is located, and show them how easy it is to check the air pressure in their tires. At some point I will have them create mini posters to hang throughout the school to advertise the ways to conserve when using an automobile.
Lesson 2: Ethanol Production and the Pros and Cons of Biofuels
This lesson will entail the students gaining an understanding of "Carbon Neutrality", and how biofuels could reduce the carbon emissions associated with motor fuel. First I will introduce them to the equations for photosynthesis and combustion, so they can see that they are inversely related. We will discuss the energy requirements for photosynthesis and relate this to the energy that is contained in biofuel. They will find that the biofuels are basically just stored sunlight energy.
The class will have already been presented on articles and data surrounding the effects of corn farming in the mid-west on environmental issues such as the "Dead Zone" in the Gulf of Mexico.
Activity 1: The Pros and Cons of Corn Ethanol
We will revisit the concept of Environmental Unity, and they will have to write some sort of opinion paper on the issues discussed in class. One possible activity is to have the class debate the merits of corn ethanol. Having debates is a good way to get students to research and understand both sides of an issue. One of the articles that students will read centers on the EROI of corn ethanol. We will discuss the energy efficiencies discussed, and pose questions regarding the use of corn ethanol.
Activity 2: Enzymatic Degradation of Cellulose
The last activity in this part of the lesson will involve the students attempting to degrade cellulose using cellulase, a common enzyme available from most biological supply companies. Students will use the enzyme on two different forms of cellulose; processed cellulose (white paper), and unprocessed cellulose (Switchgrass stalks). I will provide each group with the supplies, including glucose test strips. Since cellulose is a polymer of glucose subunits, once the cellulose is degraded the glucose test strips will indicate the presence of sugars. The glucose test strips are commonly available at most drug stores, or from biological supply companies.
The basic procedure that I have developed is as follows:
1) Prepare 200-300 ml of a 5% cellulase stock solution.
2) Give each group of students 2 vials with caps. Vials should each hold about 20 ml of solution. Have students mark the vials appropriately.
3) Provide each group with a few strips of printer paper, or the "dots" from a paper punch, as well as a few stalks of switch grass or corn husk.
4) Students should put paper in one vial and switchgrass in the other, taking care to put approximately the same amount in each.
5) Students should add about 20 ml of the cellulase solution, and test both vials for glucose concentration using the glucose test strips.
6) Let the vials sit for about 2 days. Then have students record visual data, as well as glucose concentration from a second set of glucose test strips.
I will show the students an electron microscope image of the cellulose in ordinary sheets of printer paper, and have them view ordinary printer paper through a light microscope to demonstrate the processed nature of the cellulose in paper products. We will also discuss in class the methods used in the paper industry to create paper from wood pulp. I will give them an article about paper processing so they can relate it to the processing of cellulose for ethanol.
This activity will also present a good opportunity to reinforce good scientific technique, with the inclusion of control groups, etc. I may also extend the lesson and demonstrate the denaturation of an enzyme by heating a small sample and using it on a sample of the paper strips. This will be a good springboard to explain the relatively fragile nature of some enzymes, which will eventually lead to our final lesson concerning biobuanol and its production.
Lesson 3: Biobutanol vs. Bioethanol
The final lesson in the series involves students once again calculating total bond energies and making comparisons. I will have them do an activity similar to the activity where they calculated the carbon dioxide emissions from the combustion of gasoline. I will give them structural diagrams of both ethanol and butanol, and have them calculate the relative amounts of energy contained within the molecules (See Appendix 3). After they have all agreed on calculated energy content numbers, we will combust known volumes of both alcohols and determine the energy output by using my set of classroom calorimeters.
A calorimeter measures the heat energy of a combustible product by measuring the temperature rise in a vessel of water. The students then can relate the relative temperature differences and get a fairly good approximation of the difference in chemical energy released by the combustion process. They will be able to relate the energy content of the ethanol to the energy content of butanol. This lab will once again reinforce good lab practices. After they have derived their experimental data, I will reveal the actual numbers and we will see how close they came to the actual energy content numbers. We may then discuss the sources of error, and how we could design a more rigorous experiment. This type of after-lab reasoning is very good for students to learn. Too often they think that the lab is over once they have a number and have filled in some worksheet. I try to emphasize to them that science is ever-evolving, no experiment is perfect, and there are always things that could be done to improve techniques and data collection methods.
The students will also get some articles that outline the production of biobutanol using strains of bacteria rather than enzymes. We will have a discussion of the relative merits of the different biofuels, their production, and their carbon footprint, and students will ultimately be required to write a position paper, and defend a certain fuel using the data and research that have gathered. I typically assign these types of position papers as a way to get the students to really think about all of the implications of a topic.
Appendix 1
Standards Addressed
Since I teach this class as an AP class, I apply the standards of the College Board. The College Board standards that relate to this curriculum are as follows:
Energy Resources and Consumption
A. Energy Concepts (Energy forms; power; units; conversions; Laws of Thermodynamics)
B. Fossil Fuel Resources and Use (Formation of coal, oil, and natural gas; extraction/purification methods; world reserves and global demand; synfuels; environmental advantages/disadvantages of sources)
C. Renewable Energy (Solar energy; solar electricity; hydrogen fuel cells; biomass; wind energy;small-scale hydroelectric; ocean waves and tidal energy; geothermal; environmentaladvantages/disadvantages)
I also have applied several standards from the State of Delaware, as they also apply to my curriculum unit.
Delaware Science Standard 2
Materials and Their Properties
Enduring Understanding: Materials' properties determine their use. New materials can improve the quality of life.
Students will be able to explain how development and production often raise social, economic, and environmental issues that require analyses of the risks and benefits.
Delaware Science Standard 8
Ecology
Enduring Understanding: Organisms and their environments are interconnected. Changes in one part of the system will affect other parts of the system.
Students should be able to explain how feedback loops keep an ecosystem (at the local and global level) in a state of dynamic equilibrium (e.g., positive and negative feedback loops associated with global climate).
Enduring Understanding: Humans can alter the living and non-living factors within an ecosystem, thereby creating changes to the overall system.
Students should be able to research and discuss ways in which humans use technology to reduce the negative impact of human activity on the environment. (e.g., phytoremediation, smokestack scrubbers).
Appendix 2
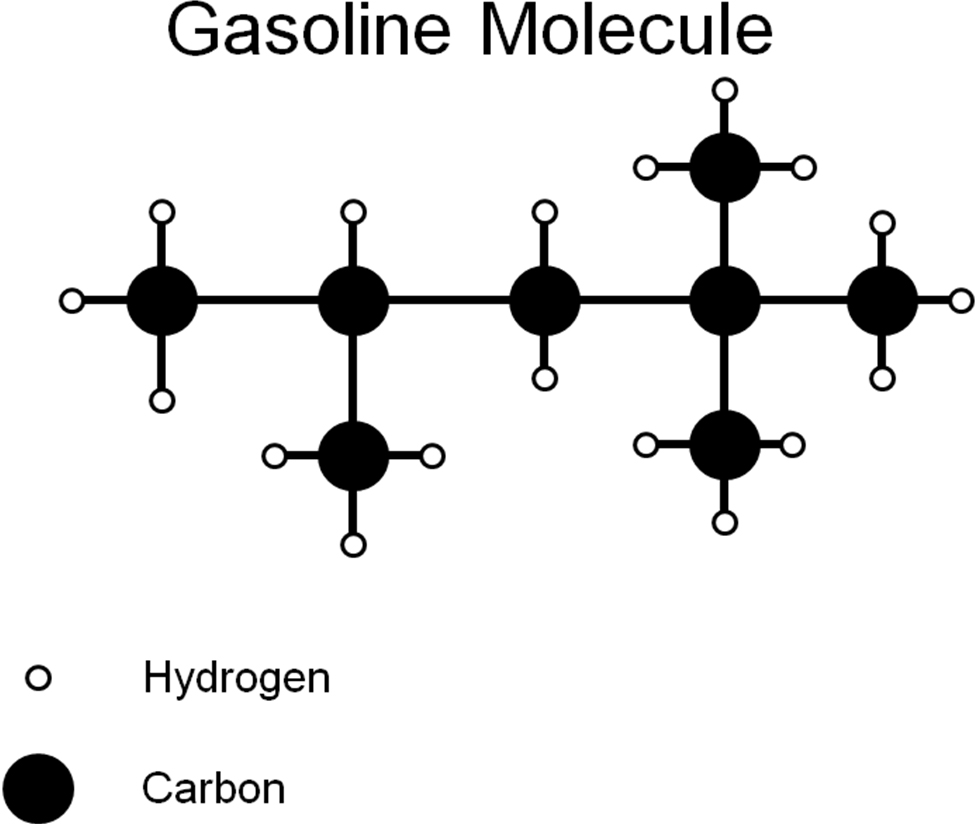
Fundamental Ideas:
1) Write a reaction for the complete combustion of gasoline to carbon dioxide and water.
2) Balance the equation based on the Costello Law of Thermodynamics
3) Explain in terms of the balanced equation how much carbon dioxide is produced per molecule of gasoline.
4) Based on atomic masses, explain the mass of carbon dioxide produced per mole of gasoline.
5) If you combusted 1000 g of gasoline completely, what is the mass of the carbon dioxide that would be produced? What is the mass of water vapor produced?
6) If gasoline weighs 6.073 lbs/gallon, and you burn 15 gallons of gasoline, how much carbon dioxide have you added to the atmosphere, assuming 100% combustion?
Appendix 3
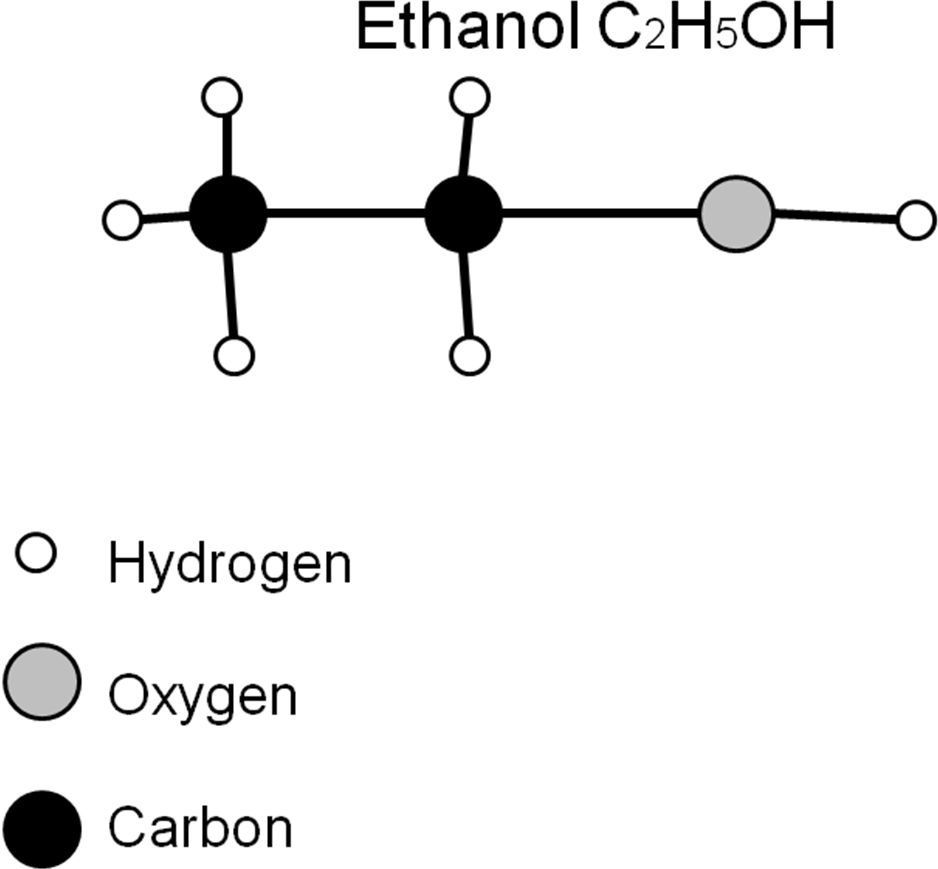
Average Bond Energies (kJ/mole)
C-C 348
C-H 413
C-O 358
O-H 463
Fundamental Ideas:
1) Using the above table of bond energies, calculate the total energy within one mole of Ethanol and one mole of Butanol.
2) Describe the relative amounts of energy as a ratio.
3) Compare the calculated energy content of Ethanol and Butanol to that of Gasoline.
4) Explain why one of the biofuels would be a better replacement for gasoline, based on energy content per mole.
Works Cited
Crowley, T.J.. "Causes of Climate Change Over the Past 1000 Years." Science 289 (2000): 270-277.
Costello, Christine, W. Michael Griffin, Amy E. Landis, and H. Scott Matthews. "Impact Of Biofuel Crop Production On The Formation Of Hypoxia In The Gulf Of Mexico." Environmental Science & Technology 43, no. 20 (2009): 7985-7991.
Schill, Susanne Retka. "Ethanol Producer Magazine | EthanolProducer.com." Ethanol Producer Magazine | EthanolProducer.com. http://ethanolproducer.com/articles/9195/gm-ford-announce-e15-compatibility-with-new-models (accessed July 10, 2013).
West, Larry. "Ethanol Production - How is Ethanol Made?." Environmental Issues - News and Information about the Environment. http://environment.about.com/od/ethanolfaq/f/ethanol_process.htm (accessed July 10, 2013).
Buckeridge, Marcos S., Amanda P. Souza, Rebecca A. Arundale, Kristina J. Anderson-Teixeira, and Evan DeLucia. "Ethanol From Sugarcane In Brazil: A âéËœmidwayâé™ Strategy For Increasing Ethanol Production While Maximizing Environmental Benefits." GCB Bioenergy 10 (2011): 1707-1757.
Farrell, A. E.. "Ethanol Can Contribute To Energy And Environmental Goals." Science 311, no. 5760 (2006): 506-508.
Gasparatos, A. , P. Stromberg, and K. Takeuchi. "Sustainability impacts of first-generation biofuels." Animal Frontiers 3 (2013): 12-26.
Tilman, David, Kenneth G. Cassman, Pamela A. Matson, Rosamond Naylor, and Stephen Polasky. "Agricultural Sustainability And Intensive Production Practices." Nature 418, no. 6898 (2002): 671-677.
Gasparatos, Alexandros. "Water for Bioenergy : A Global Analysis." In Socioeconomic and environmental impacts of biofuels: evidence from developing nations. Cambridge: Cambridge University Press, 2012. 69-89.
Searchinger, T., R. Heimlich, R. A. Houghton, F. Dong, A. Elobeid, J. Fabiosa, S. Tokgoz, D. Hayes, and T.-H. Yu. "Use of U.S. Croplands for Biofuels Increases Greenhouse Gases through Emissions from Land-Use Change." Science 319, no. 5867 (2008): 1238-1240.
Young, Raymond Allen. Cellulose: structure, modification, and hydrolysis. New York: Wiley, 1986.
Blanco-Canqui, Humberto. "Energy Crops And Their Implications On Soil And Environment." Agronomy Journal 102, no. 2 (2010): 403.
Klemm, Dieter, Brigitte Heublein, Hans-Peter Fink, and Andreas Bohn. "Cellulose: Fascinating Biopolymer And Sustainable Raw Material." ChemInform 36, no. 36 (2005): 67-74.
Adler, Paul R., Stephen J. Del Grosso, and William J. Parton. "Life-Cycle Assessment Of Net Greenhouse-Gas Flux For Bioenergy Cropping Systems." Ecological Applications 17, no. 3 (2007): 675-691.
Varvel, G. E., and W. W. Wilhelm. "Soil Carbon Levels In Irrigated Western Corn Belt Rotations." Agronomy Journal 100, no. 4 (2008): 1180-1184.
Mussatto, Solange I., Marcela Fernandes, Adriane M.F. Milagres, and Ina C. Roberto. "Effect Of Hemicellulose And Lignin On Enzymatic Hydrolysis Of Cellulose From Brewer's Spent Grain." Enzyme and Microbial Technology 43, no. 2 (2008): 124-129.
Hess, J. Richard, Kevin L. Kenney, Christopher T. Wright, Robert Perlack, and Anthony Turhollow. "Corn Stover Availability For Biomass Conversion: Situation Analysis." Cellulose 16, no. 4 (2009): 599-619.
Pfromm, Peter, Vincent Amanor-Boadu, Richard Nelson, Praveen Vadlani, and Ronald Madl. "Bio-butanol vs. bio-ethanol: A technical and economic assessment for corn and switchgrass fermented by yeast or Clostridium acetobutylicum." Biomass and Bioenergy 34 (2010): 515-524.
Dhamole, Pradip , Zhilong Wang, Yuanqin Liu, Bin Wang, and Hao Feng. "Extractive fermentation with non-ionic surfactants to enhance butanol production. ." Biomass and Bioenergy 40 (2012): 112-119.
Mullin, David. "Green Car Congress: Novel bacterium produces butanol directly from cellulose." Green Car Congress. http://www.greencarcongress.com/2011/08/tu103-20110828.html (accessed July 10, 2013).
Bond-Watts, Brooks B, Robert J Bellerose, and Michelle C Y Chang. "Enzyme Mechanism As A Kinetic Control Element For Designing Synthetic Biofuel Pathways." Nature chemical biology 10 (2011): 537.
Davis, Sarah, William Parton, Stephen Del Grosso, Cindy Keough, Ernest Marx, Paul Adler, and Evan DeLucia. "Impact of second-generation biofuel agriculture on greenhouse-gas emissions in the corn-growing regions of the US." Frontiers in Ecology and the Environment 10 (2011): 69-74.
Comments (0)
THANK YOU — your feedback is very important to us! Give Feedback
