Introduction & Rationale
“Energy and persistence conquer all things.” – Benjamin Franklin
The advent of the Industrial Revolution in 1769 forever altered our way of life and reliance on energy. Only two-hundred and fifty years ago, the world was introduced to light bulbs, steam engines and locomotives. Globally, the human population exploded as did the demand for energy. At no other time in human history have we relied on energy as we do now. Take for example one of the most sought after forms of energy, electricity. We use it to power our cellphones, computers, lights, and appliances. Today, electricity is essential for developing countries as it ensures economic growth and fosters innovation critical for technological progression. How we generate electricity varies with geopolitics, access to technology, economics, and proximity to resources. Historically we have consumed a variety of non-renewable energy resources such as coal, petroleum oil and natural gas. Today countries are expanding their energy portfolios by investing in renewable technologies that include solar, wind, biomass, tidal, and geothermal as a way to build sustainable capacity. In order to understand our current sustainable energy crisis we must ask several fundamental questions: what is energy, how much energy do we consume, what are the effects of non-sustainable energy sources and what renewable energy resources exist in the United States.
Energy is a property of an object that can be transferred to other objects or be converted into different forms. XV Energy’s inherent flexibility coupled with its conservative nature allows us to harness power to perform work. Since the Industrial Revolution, society has relied upon fossil fuels – mainly coal, natural gas and oil – to convert highly energetic hydrocarbons into heat and electricity. These stored sources of fuel were once organic matter in plants that harvested solar energy. Over millennia the organic matter transformed, through heat and pressure, into our current sources of fossil fuels. As populations have increased so too has our demand for energy.
According to data published by International Energy Agency (IEA) the yearly global consumption of energy was 143,851 TWh in 2008, with the U.S. contributing 29,014 TWh. XVI To truly grasp the scale of energy consumption, let’s look at some examples we can relate to. A single fluorescent light bulb is powered by twenty-seven watts. Remember a watt is the amount of work performed by energy per unit of time, in this case the work done is to provide light. An external air conditioner unit typically consumes 1.4 kW. If we were to install 1 billion of these air conditioners and run them all the time for an entire year, we would consume 12,264 TWh (i.e. 1.4 TW times 8760 hours per year). This would account for only about 42% of our annual consumption, which is continuing to rise to meet economic demands. Data from the IEA shows that the average energy use per person increased 10% from 1990 to 2008. XVI These energetic trends are expected to rise as emerging economies enter the world market. One of the most rapidly growing economies is China, which has doubled its energy consumption. In 2001, China consumed an average of 979.25 kg oil equivalent per person in a year, and by 2011 it surged to 2,029.36 kg oil equivalent. XVI In 2008, China consumed more total energy than the United States, primarily from coal-fired power plants. Today, China is diversifying its energy portfolio by investing in renewables such as solar, wind, and hydroelectric. Likewise, the U.S. and members of the European Union are funding development of renewable technologies and enhancing energy efficiency in existing infrastructure to reduce greenhouse gas emissions. The U.S. government has provided a variety of subsidies to companies in the hopes of developing a competitive market for renewable energy. Tremendous progress is still needed to overcome our reliance on fossil fuels and reduce emissions.
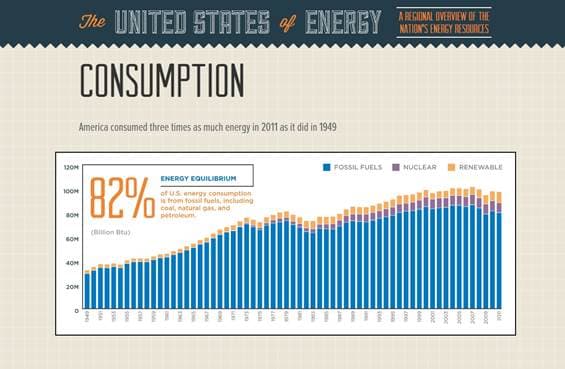
Figure 1: “The United States of Energy” created by Saxum illustrates the rapid increase in energy consumption from 1949 to 2011 in the United States.
The scale of the energy market has led to measurable impacts on the planet. In 2010, the world’s greenhouse gas emissions were approximately 9,500 million metric tons of CO2 from fossil fuels. XII Carbon dioxide, a green-house gas, prevents the infrared spectrum from escaping Earth’s atmosphere leading to the overall warming of the global climate. The latest Intergovernmental Panel on Climate Change (IPCC) report produced several comprehensive predictive models that illustrate the dire need to cut emission levels by 40-70% before 2050. XVII Scientists estimate a global rise in temperature of approximately 2 ºC with some estimates up to 5 ºC. XVII These marginal temperature increases over a short amount of time may have disastrous consequences to national economies, food security, ecological diversity, and more importantly our children’s future. Unless active discussions and agreements between countries should arise or global consciousness takes precedent over individual needs, the future remains precarious. The G20 countries, the major contributors of carbon emissions, have tentatively agreed to reduce emission levels. XVII However, global energy consumption continues to rise as North American and Asian economies continue to grow with the complex globalized marketplace.
Globalization has inextricably linked the world together, thus making energy security and climate change an international issue. Historically, increased energy demand has led to geopolitical conflict, improved efficiencies and technological innovation. We can look back and cite several examples from that past that demonstrate this phenomenon. The invasion of Iraqi forces and occupation of Kuwait led to the Gulf War in 1990-1991, a direct result of energy security. At the time, Saddam Hussein implored OPEC to reduce overall production to increase the price of oil. As a petro state, Iraq was dependent upon the revenue of oil exports. Kuwaiti officials rejected Saddam’s proposal, which inevitably led to the invasion, resulting in the Gulf War. Another example of geopolitical conflict arose when the United States provided aid to Israel in the 1973 Yom Kippur War. The U.S. supplied arms to Israel after Egypt and Syria launched a military campaign against Israel. As a response, OPEC along with other Arabic nations enacted an oil embargo against the U.S. The 1970’s oil crisis led to surging prices, from $3 per barrel to $12 per barrel within a year. VII By the 1970’s, U.S. oil production was already declining which further compounded the oil shortage. The regional vulnerability of the Middle East has increased domestic energy efficiency. Throughout periods of high oil prices, energy efficiency gains have manifested in a number of ways, most notably in car efficiency standards. In 1985, Congress passed a law that required cars to have a minimum level fuel efficiency to prevent future oil shocks. VII After 2008 when oil prices peaked at $136.1 per barrel, the Obama administration introduced a bill that required increased car efficiency standards from 30 mpg to 54.5 mpg by 2025. VII In addition to the investments in energy efficiencies, the U.S. has spent millions on innovations in renewable technology to reduce CO2 emissions. The U.S. is poised to become a global leader in renewable technology due to its bountiful carbon-free resources around the country: from the untapped solar energy in the southwest, to the sprawling wind belt throughout the Midwest (Figure 2). Throughout the 1990’s, California fully embraced renewable energy with numerous wind farms and solar installations. These controversial projects were funded through government subsidies after the Gulf War. The initial investment was fraught with expensive wind turbines that intermittently produced power. Technological optimization of wind energy has now become a viable alternative to fossil fuels. By 2015, California generated 12,000 GWh of wind energy, which is double the capacity 5 years ago. VII Other states are in the process of assessing their environmental assets to minimize their carbon footprint and gain energy independence. Innovations continue to emerge from the energy crisis but the environmental crisis that we face may push the limits of human innovation.
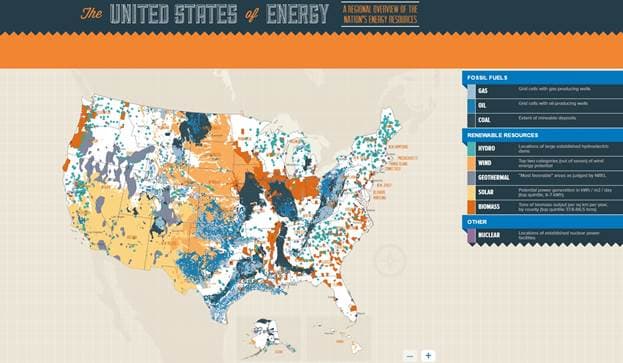
Figure 2: “The United States of Energy” created by Saxum depicts the regional energy resources within the United States.
One innovation that has become popularized by the United Sates and Brazil is biofuel energy. These energy sources are derived from biomass resources that include corn, sugarcane and switch grass for the production of ethanol. This alternative fuel source comes from the fermentation of simple sugars derived from plants. One of the highest sugar-content crops is sugarcane which is has been a staple crop produced in Brazil. Throughout the early 1970’s, Brazil was importing 85 percent of its oil. VII This came to an abrupt halt with the 1973 oil crisis, when the price of oil tripled. VII As a result, the Brazilian government launched a Pro-Alcohol program to manufacture ethanol from the surplus of sugarcane crops. The program was so successful that Brazilians now drive fuel flex cars and motorcycles that allow them to alternate between traditional petroleum based products and ethanol. In 2010, 6.9 billion gallons of ethanol fuel were produced. VII In the United States, innovation was prompted with the 2008 recession, when oil prices surged, forcing many to be resourceful. Small business owners began harvesting used vegetable oil from restaurants to produce biodiesel. This alternative fuel source is manufactured through a process called transesterification, where the glycerin is separated from the fatty acids. This application is a rather simple process but is limited by reliable sources of surplus vegetable oil. Innovations like this coupled with optimizing efficiency will be imperative for cutting emission and solving the energy crisis in the future. However, we must commit to our investments for the future, even when prices of oil remain low.
In this unit, we will explore how biodiesel plays a role in alternative energies and integrate fundamental energetic principles to inform our students about the limitations of energy transformation. The energy and environmental crisis of the 21st century is vital for students to comprehend and participate in. Policies of today will directly impact their future, as they inherit a globalized economy that will require diplomatic compromise and international collaboration. This unit introduces learners to the unsustainable energy crisis while simultaneously reinforcing the physical concepts associated with energy using biodiesel as a vector of inquiry. It is imperative that students develop strong foundational skills in physical science so that future innovations can be realized.
School Demographics
Ballou High School is a low-income, low-performing school with approximately 678 African American students, located in the southeast of Washington DC. Here gun violence and drug trafficking are prevalent, fueled by gang activity. Violence is a way of life in southeast DC and many students experience emotional and physical trauma, often leading to volatile behavior in the classroom. Many students struggle to get to school due to trouble at home or with the law. As a result, truancy is high at Ballou High School, with approximately 68% (32% absent) in seat-attendance. In addition, high occurrences of teen pregnancy lead to further time away from school. Students who lack consistency often fall behind in class and cause behavioral disruption further impeding potential educational gains. Students typically score below grade level in math and reading. Initiatives have been implemented to curtail these trends; however, it will take several years to see academic gains. The students at Ballou High School learn best with a positive, structured, dynamic classroom, with hands-on activities coupled with praise or rewards. Many students have not seen academic success and yearn to learn. The biodiesel project will excite many students about the possibilities of physics and finding a voice within the community. Relating the material to what interests them will allow better buy-in overall. This will fuel students to meet the high academic expectation set at the school and in the classroom. Students will get opportunities to collaborate and communicate their results to the community. In addition, they will have a valuable skill that would look good for possible biotech employment. When preparing students for the 21st century it is essential to introduce topics as they pertain to the community directly, in addition to the global impacts. The project is but a pebble in an ocean but its implementation may have rippling effects across that school and community.

Comments: