- Login
- Home
- About the Initiative
-
Curricular Resources
- Topical Index of Curriculum Units
- View Topical Index of Curriculum Units
- Search Curricular Resources
- View Volumes of Curriculum Units from National Seminars
- Find Curriculum Units Written in Seminars Led by Yale Faculty
- Find Curriculum Units Written by Teachers in National Seminars
- Browse Curriculum Units Developed in Teachers Institutes
- On Common Ground
- Publications
- League of Institutes
- Video Programs
- Contact
Have a suggestion to improve this page?
To leave a general comment about our Web site, please click here
Confronting the Plastic Wasteland through Engineering
byTaissa LauIntroduction
Look around you and try to identify anything that is made of or contains plastic. Notice your car, laptop, phone, clothes, food packaging, children’s toys, etc.; all of which contain some sort of plastic. Plastic is a necessary material for our modern-day living. However, would you be surprised to find out that plastic’s popularity only just began to rise in the late 1950s? As you can see in Figure 1 below, the production of plastic has grown exponentially since 1950. The favorable characteristics of this synthetic polymer are what makes its use so widespread. The terms “synthetic” and “polymer”, when used to describe plastic, can most simply be defined as many chemical units repeated and put together. Each type of polymer has its own unique characteristics, but most plastics share some similarities. Plastics are typically low density, inexpensive, durable, can be processed in many different ways depending on the function it will serve, and they can be used to make products not accessible in the natural world.1 The allure of this synthetic material is enticing, but there is a caveat. Plastics are not biodegradable, meaning they cannot breakdown completely and can thus accumulate all over our world.

Figure 1. Graph depicitng plastic production in metric tons per year.2
The rise in plastic production grew from two metric tons in 1920 to 380 metric tons in 2015.3 In 2017, the EPA reported the average person generated four and half pounds of waste every day. As a nation, the U.S. generated approximately 268 million tons of waste in that year. Of those 268 million tons, plastic made up about 13% or 35 million tons. When we only consider plastic waste, 75% was placed into landfills or ended up in the natural environment, 16% was combusted with energy recovery, and only 8% was recycled.4 A visual representation of the plastics waste management is provided in Figure 2 below.

Figure 2. Graph depicting plastic waste management methods by tons.5
If the trends of plastic production and waste continue at the this rate, about 12,000 million metric tons of plastic waste will accumulate in landfills or the natural environment by 2050.6 The contamination of the natural environment with plastic waste should be of paramount concern. When plastic waste enters the environment, whether it be a landfill or as litter, UV exposure and mechanical degradation will break down larger plastic items into smaller microplastics, which range micrometers to millimeters in size.
“Microplastics are of special concern since their bioaccumulation potential increases with decreasing size. Microplastics may be ingested by various organisms ranging from plankton and fish to birds and even mammals, and accumulate throughout the acquatic food web. In addition, plastics contain a multitude of chemical additives and absorb organic contaminants from the surrounding media. Since these compounds can transfer to organisms upon ingestion, micorplastics act as vectors for other organic polluntants and are, therefore, a source of wildlife exposure to these chemicals.”7
While the numerous beneficial characteristics of plastics make them a necessity for today’s society, those same characteristics are now presenting many challenges to environmental health. Countries are responding to those challenges with new environmentally-friendly policies, initiatives, and engineering. Within this unit, there are three overarching goals to be achieved. First, it aims to address the basic chemistry of plastics exploring the basic chemical synthesis of polymers. Second, the life cycle of petroleum-based plastics is addressed, which will reveal the challenges of plastic waste. Third, students will consider the future of plastics utilizing their newly acquired knowledge of current pletroleum-based plastic production and disposal. They will address the need for more sustainable practices in synthesizing plastics by designing, creating and testing their very own bio-based, biodegradable plastic or repurposing petroleum-based plastic in another form.
Rationale and School Demographics
The content of this unit is designed to be used in alignment with the engineering standards of the Next Generation Science Standards (NGSS). The NGSS were adopted by the state of Illinois for use in science education in 2014. Since that time, science instruction is becoming more commonplace within my school, Tarkington School of Excellence, however one area still falling short is students’ engagement in engineering. Tarkington is a Title I school located on the southwest side of Chicago, Illinois. Tarkington serves the community as a public neighborhood school and as a training site for preservice teachers in the Academy of Urban School Leadership. It is made up of approximately 1,000 students in kindergarten through eighth grade, and 93% of its students are considered low income. The demographics that make up the student body are approximately 77% Hispanic and 22% Black students. This unit was designed to be delivered to an eighth-grade student body made up of 115 students. Of the 115 students, 15% receive Special Education services and 23% are English Language Learners.
As of the 2019-2020 school year, Tarkington School of Excellence required kindergarten through fifth-grade classes to teach Science weekly. This was a change from previous school years in which Science was only recommended to be taught weekly in those grade levels. Students begin taking Science as a core content class beginning in sixth grade when they receive daily science instruction. In middle school, there are approximately 40 weeks in the school year and science teachers only spend three to four weeks total engaging students in engineering experiences. “Engineering can be a meaningful way to engage students’ wide range of prior experiences in STEM, helping open the field to be more culturally relevant and meaningful to young learners. It can give students opportunities to deepen their science knowledge by engaging in problem-solving around locally-relevant issues.”8 Engineers solve problems and design devices and infrastructure. It is important to expose students to engineering since it will deepen their critical thinking skills and allow them to apply the science content they learn over the course of the year to new applications. This unit can integrate many different scientific concepts from any area of science, depending on what an educator would like it to complement in their course of teaching. The content of this unit could be easily adapted for any middle grade level, and it has enough content that could make it relevant to any strand of science education.
Content Objectives
The initial objective of this unit is to present the science behind plastic production. Students will investigate the basic chemistry of plastics by understanding the types of bonds produced in synthesizing polymers. Understanding the types of bonds found in synthetic polymers will establish students’ background knowledge on why plastics disposal is such a challenge. The second objective of this unit addresses the life cycle of plastics from raw materials to disposal. From this experience, students will reveal the challenges of plastic waste, the current state of plastic pollution in natural environments like the Great Lakes, and the current known effects of plastic pollution on ecosystems. This unit will provide information on plastic pollution in the Great Lakes since this has a direct impact on our community in Chicago, but depending on your location a more relevant ecosystem could be selected for investigation for your students. The final objective of this unit is to provide an experience in the engineering design process as students develop their own biobased, biodegradable plastic or repurpose used petroleum-based plastic for some other everyday use.
Content Background
The Plastic Revolution
Natural polymers were first explored in the late 19th century, most notably cellulose. Scientists were able to modify cellulose to produce a polymer that was eventually used in things like hair brushes, jewelry, toothbrushes and cinematographic film due to its easily moldable characteristics. This polymer became known as Celluloid and is regarded as the first synthetic plastic material.9 In 1906, another scientist, Leo Baekeland, experimented with phenol and formaldehyde mixtures. In 1909, he announced his invention which he coined as Bakelite, an easily moldable and less expensive alternative to Celluloid made up of a phenol-aldehydes, creating a thermoset plastic.10 By 1910, he began his company called the General Bakelite Company in the United States and shortly after began commerically producing his product. During the 1930-1940 decade, four of today’s major thermoplastics were developed: polyvinylcholoride (PVC), polystyrene (PS), polyolefins, and PMMA (acrylic). As new plastics were developed, their popularity in usage increased in various fields due to their wide variety of characteristics dependent on their chemical makeup.11
Prior to World War II, production of many thermoplastics at the time used vegetable sources as their raw material. Henry Ford even experimented with soybean oil to create the plastic panels of his soybean car. However, war brought about a high demand for new synthetic plastics, which were more economical but durable for wartime materials like parachutes, aircraft components, helmet liners, and much more. Further production and investigation of biobased sources was halted as chemists began experimenting and creating new synthetic plastics to aid the war efforts. By the end of World War II, plastic production almost quadrupled from 213 million pounds in 1939 to 818 million pounds in 1945.12 After moving away from vegetable sources due to the war, coal was a leading source of raw material in plastic production but eventually this gradually shifted to petroleum. “Today, the plastics industry is heavily integrated with the oil industry. The development of the petrochemical industry was probably the greatest single contributing factor in the growth of the plastics industry.”13plastics production in the United States grew exponentially from 390 thousand tons in 1960 to 35.3 million tons by 2017, as shown in Figure 1.14
Chemistry of Plastic
Plastics are a type of polymer, which is made up of monomers. Monomers are individual chemical units and when those units are repeated and covalently bonded, they create a polymer. In the case of plastics, they are a type of synthetic polymer created by scientists. The monomers that are most prevalent in today’s synthetic polymers, or plastic, come from raw materials like coal, oil, and natural gas.15 Not all polymers are synthetic, though. There are many examples of polymers that exist in nature. Some of those examples include starch and cellulose, which were the first type of raw materials used in the production of plastics like Celluloid and Bakelite. Most people are probably more familiar with the terms proteins and carbohydrates. Proteins and carbohydrates are natural polymers made up of long carbon frameworks. Polymeric structures, whether natural or synthetic, can be linear, branched or crosslinked describing the structure of the bonds between monomers. The chemical structure of a polymer determines what type of properties it will have.
All polymers consist of covalent bonds since they are composed of non-metallic elements. These non-metallic elements prefer to share their electrons with other elements rather than gain or lose any electrons. The sharing of electrons is what creates the covalent bond. Carbon becomes a popular element in polymers due to the number of of valence electrons available. Carbon has four valence electrons allowing it to share those electrons with other elements. Carbon also creates strong bonds with other carbon atoms. Most plastics are based off of a carbon backbone.
There are two broad classifications of plastics: thermosets and thermoplastics. Thermoplastics are plastics that can be heated up, which melts the plastic making it able to be remolded. The chemical structure of thermoplastics is either a linear or branched polymer. Linear polymers are typically long backbone chains that look like spaghetti and branched polymers are similar but with additional shorter chains equally spaced and bonded along the backbone chain. Approximately 92% of plastics are considered thermoplastics.16 Thermosets are plastics that, once molded and set, cannot be broken down into their original form. This is due in part to their chemical structure. The chemical structure of a thermoset is a crosslinked polymer. Crosslinked polymers have a structure in which multiple backbones can be connected resembling a ladder. These types of structures and bonding are very difficult to break apart. The chemical structures determine the characteristics of each plastic ever created and those characteristics are what drive the demand of plastic for use in different products.
The physical characteristics possessed by polymers can be determined by their length and their number of cross-links if applicable. Cross-linked polymers are harder to breakdown due to their more complex structure when compared to a linear polymer. The more cross-links the polymer has, the harder it is to breakdown. This type of structure presents the challenge to how humans can manage plastic waste.17
Life Cycle
For the purposes of this unit, the life cycle of petroleum-based plastics will be further investigated in this section. The most common elements in petroleum-based plastics are carbon and hydrogen. These elements come from the raw materials of oil, natural gas and coal, which are nonrenewable resources. The fact that nonrenewable resources are used in the production of petroleum-based plastics is just one of the issues to overcome for a more sustainable plastic future.
The raw materials must first undergo a process called “cracking”. When these materials are cracked, heavy hydrocarbon molecules are broken up into lighter molecules. The cracking process can be done by heat, pressure, or catalysts. Once cracking is complete, the resulting gases are hydrocarbon monomers like ethylene or propylene18. These gases can be used or processed further to create more monomers like styrene, vinyl cholride, ethylene glycol, etc.19 Next, the raw materials must undergo one of two types of polymerization reactions. Polymerization combines monomers to create polymers. One such polymerization reaction is a condensation reaction. In this type of reaction, two monomers combine and one monomer loses a hydrogen and the other monomer loses a hydroxyl. The lost hydrogen and hydroxyl combine to form water. The remaining electrons from the monomers covalently bond and form a polymer. The variation of monomers forming polymers is what allows for various characteristics of plastics.20 The second type of polymerization reaction is an addition reaction. In this type of reaction, double bonded electrons rearrange to form single bonds with other monomers.21
Additives can be used in the production of plastics. The additives can change the mechanical, physical, or chemical properties of the final plastic product. Examples of these alterations can include protection from the effects of heat, the addition of color, flame-retardancy, and much more22. Additives do not covalently bond to the other elements, which can lead to their leaching out when plastics are disposed of in the environment leading to a myriad of harmful effects on the ecosystems they enter. After additives are blended in, processing continutes with one of four main methods: extrusion, injection molding, blow molding, and rotational molding. Each of these methods involves melting plastic pellets into a liquid form and then cooling to form a final product. The desired product determines the type of processing method used.23
There are seven major plastic groups, which are identified in Figure 3 below. Sometimes, when you look at a plastic bottle or container, you might notice a number surrounded by a recycle sign in the mold of the plastic. That symbol denotes the type of plastic polymer. Each group has its own characteristics that separates it from each other. Each group, however, is made up of different products that share similar characteristics. The name of each group is derived from the type of chemical backbone of the polymer that forms the product. For example, there is polyvinyl choloride better known as PVC. PVC’s characteristics make it suitable for pipes due to its high chemical resistance, but it is also used for other products like blood bags, leather products and medical tubing.24
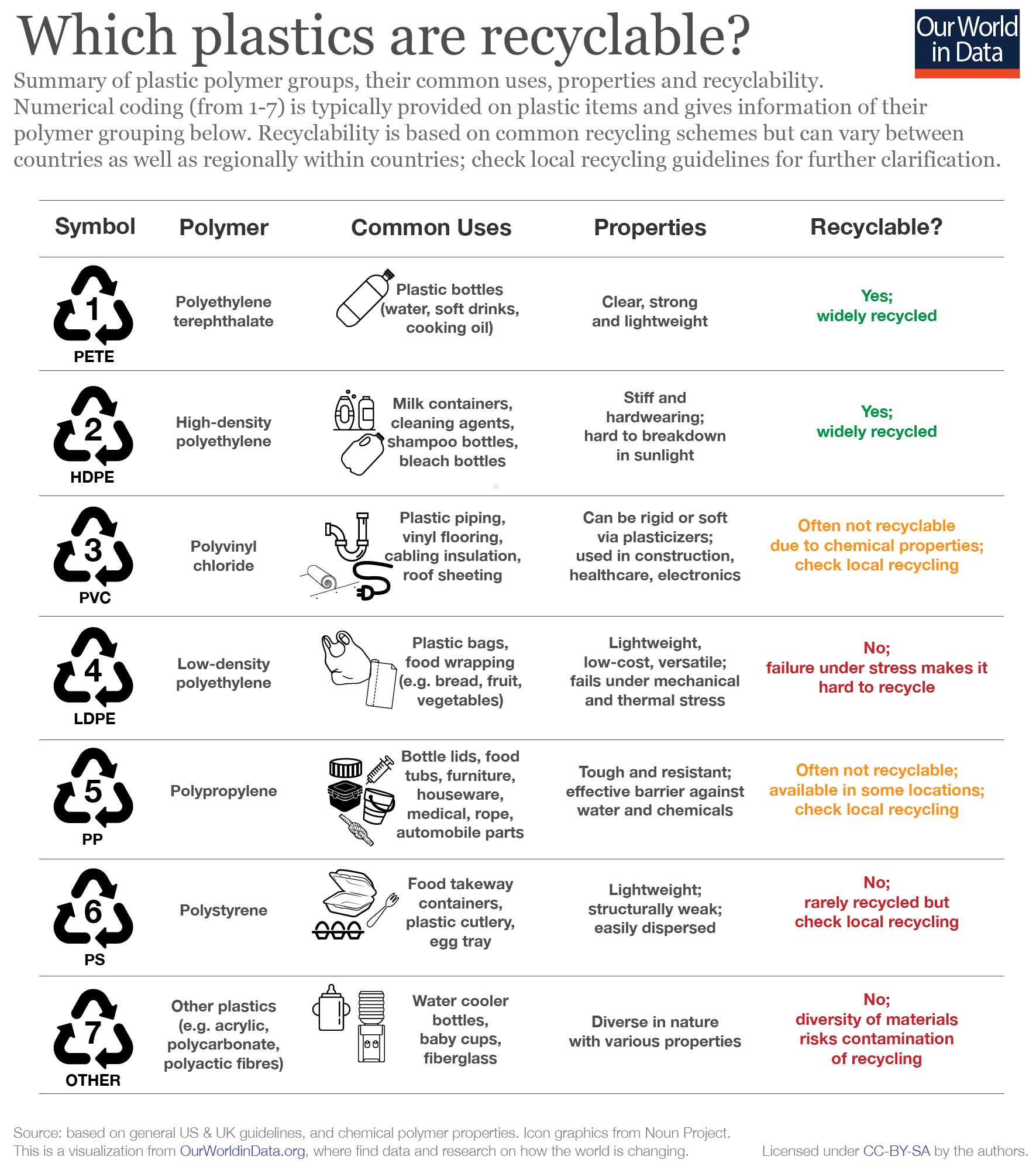
Figure 3. Chart identifying the seven major plastics groups, uses, characteristics, and recyclability.25
The end of life for plastic depends largely on what category of plastics it falls into: thermosets or thermoplastics. The fate of all types of plastic will take course on one of the following paths: deposition to a landfill, improper disposal becoming anthropogenic litter on land or in bodies of water, recycling, or incineration. Figure 4 below shows the average estimated decomposition times for common plastics that end up in a marine environment. Decomposition in this case is referring to the time it takes for these common plastic items to decompose into microplastics, which never truly leave the environment they end up in. The significant amounts of time allow for the accumulation of plastic waste, while also increasing the bioavailability of microplastic waste that remains in the environment.
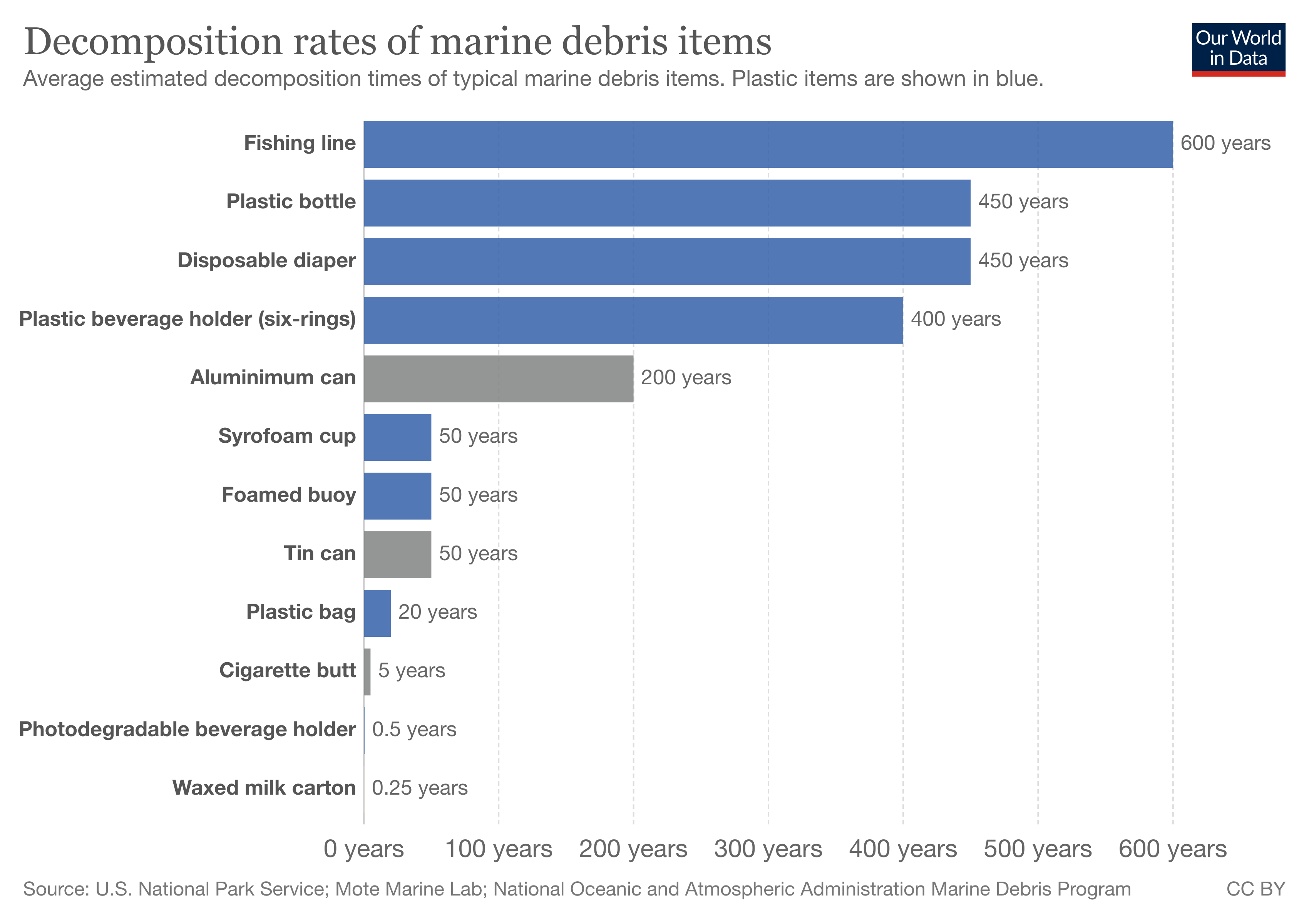
Figure 4. Graph depicting the average estimated decomposition times for items of debris.26
Recycling is another method of reducing plastic waste, however, as you can see in Figure 3, not all plastics are recyclable. Recycling programs began in the 1980s and there is a lot of skepticism of whether or not recycling works. Only 60% of the U.S. population has access to recycling programs in the form of curbside pick up or drop off recycling centers. When plastic is recycled, plastics are chopped up, washed, and sold to manufacturers to create new products.27
Another management option gaining traction is source reduction, which is defined as “activities to reduce the amount of material in products and packaging before that material enters the municipal solid waste management system.” This definition is limited in describing all the strategies that fall into this type of management, though. Source reduction activities include: redesigning products to minimize the quantity of materials used, optimizing the lifespan of products, or reusing or repurposing products that are already manufactured. This waste management option is something accessible and achievable by everyone and could be part of the solution to addressing plastic waste.28
Plastics and the Great Lakes
There are numerous studies on the accumulation and effect of plastic in marine systems, which in this case refers to the world’s major oceans, however studies of the Great Lakes and freshwater systems have not been as widespread. As plastic pollution becomes more concerning, though, there has been an increased awareness of studying the Great Lakes and more attention and review of the plastics accumulating in these ecosystems is being undertaken.
Summertime in Chicago is always highly anticipated each year after the brutal Midwest winters. Lake Michigan is arguably the most popular and busiest destination once sunny, summer days arrive. Residents flood the Lakeshore Trail running, cycling, rollerblading, etc. to enjoy and embrace the magnificent views that the lakefront offers. Families and individuals flock to Lake Michigan every Memorial Day weekend when the public beaches officially open. Kids and adults alike wade in the cool waters to escape the summer heat. What beachgoers may not realize is they are really swimming in somewhat of a plastic wasteland.
According to research performed at the Rochester Institute of Technology, of the 22 million tons of plastic waste entering the Great Lakes every year, 11 million tons of that plastic enters Lake Michigan alone. Lake Michigan receives the most plastic pollution of any of the five Great Lakes. To put it into perspective, imagine 100 Olympic-sized swimming pools filled with plastic water bottles being dumped into the lake each year.29 It is also estimated that 80% of litter found on the shorelines of the Great Lakes is plastic.30
According to a review of the plastic debris in the Great Lakes, survey data revealed that urban areas heavy with human and industrial activity, like Chicago, were most associated with higher concentrations of plastic debris.31 Some of the most abundant data about beach pollution along the Great Lakes comes from The Alliance for the Great Lakes Adopt-A-Beach program (AAB), which is a non-governmental organization made up of volunteers who remove debris from the environment and test water quality to assess general beach health. According to the AAB data collection, in 2012 about 77%-90% of the total shoreline debris collected was made up of plastic debris items.32 There are 24 public beaches on the Chicago lakefront spanning 26 miles along the shorelines of Lake Michigan.33 In a collection done at a north side beach in Chicago in 2018, a local volunteer group made up of students for the AAB program reportedly collected 86 pounds of litter in a one-day cleanup.34 It was not noted how much of that litter contained plastic debris, but the number is still striking. AAB has also indicated that in the data of plastic debris they collected from 2003-2014 it shows cigarette filters and plastic food wrappers and containers were the most commonly reported litter collected; implying that beach-goers could be a major factor in macroplastic debris.35 Another study also identified plastic resin pellets from plastic manufacturing as a major pollutant along the Great Lakes shorelines. This was linked to spillage during transport, eventually entering streams and storm sewers, which could be discharged into rivers eventually ending in the Great Lakes. Additionally, a study completed by Hoellein et. al (2014) analyzing anthropogenic debris in the Chicago River also suggested that rivers could represent a major transport pathway of plastic debris to the Great Lakes. Debris counts from this study were reported between 0-34 items/m2.36 Plastic debris can be classified into primary and secondary debris. Primary plastic debris is in its original form or close to it, and secondary plastic debris is plastic that has mechanically broken down into smaller pieces. Both types of debris have been found in Lake Michigan and the other Great Lakes. The effects any kind of debris has on an aquatic environment can impose health risks to aquatic animals due to ingestion or entanglement. Plastics are not biodegradable, thus do not degrade to carbon dioxide in the environment like other forms of organic matter. Over time, primary plastic structures are disrupted by mechanical forces and UV radiation resulting in smaller microplastics. Microplastics have high surface area to volume ratios and can release toxic plasticizers or additives including phthalates, bisphenol A, etc. from the original products that were synthesized to create the plastic. If fish, turtles, birds etc., ingest these toxins, they can mimic hormones and impact endocrine function and cause harmful reproductive and developmental effects to the animal and as a result affect the food web.37
In the Great Lakes, one team of scientists found 4,270 microplastics particles per kilogram of dry weight sediment in lake sediment, and up to 2,444 microplastic particles per kilogram in river sediment.38 Microplastics are generally defined as any plastic less than 5 millimeters but greater than 333 nanometers in diameter. Any plastic debris smaller than 333 nanometers is termed “microscopic plastic debris”. Common shapes of plastic debris include fragments, films, pellets, lines, fibers, filaments, and granules. The type of polymer determines the density. Some polymers are more dense than water and they sink into a body of water, and some polymers are less dense than water and they float. Macroplastic debris, plastic greater than 5 millimeters diameter, can breakdown into microplastics due to mechanical weathering, UV radiation, and some biodegradation however it is does not completely mineralize and these degradation processes can take hundreds to thousands of years.39 Microplastics are also produced in an original form. Microplastics that are directly produced are used as the resin pellets for producing macroplastics or in personal care and cleaning products as an abrasive. In 2015, the United States Congress passed the Microbead-Free Waters Act of 2015 banning the production and distribution of microbeads in cosmetics and non-prescription drugs like toothpastes. This law was developed out of direct concern of microbeads in the water supply and to follow suit with several states that had already banned their use.40 Some countries have similar legislation, but this initiative has not been adopted worldwide. While this is a very small beginning step to acknowledging human plastic waste, it is not even close to addressing the 360 million metric tons produced globally.
Microplastics can pose a serious threat to aquatic ecosystems. Most studies on the effects of microplastics have only studied the effect on oceanic life leaving the need for freshwater systems to be further investigated. In studies of the effects of microplastics in marine environments, there have been three notable findings. First, several aquatic species have been found to ingest microplastics allowing for bioaccumulation. It has been confirmed that ingested plastics can transfer trophic levels via aquatic food webs posing a threat to the entire ecosystem.41 Second, some plastics are developed with toxic additives, like phthatlates, bisphenol A (BPA), and polybrominated diphenyl ethers, which leads to the next finding. Plastic polymers themselves are not toxic, but the toxic additives can leach out especially as plastic debris slowly degrades in the environment. Again, as the toxins, which are not covalently bonded to the polymers, are leached into the environment, it can cause serious health effects to the environment and animals ingesting the plastic.42 Third, plastic debris can act as a vector for non-native species and pathogens. Microbial communities are different from the surrounding water suggesting the microplastic can serve as a new habitat for microbial growth and replication. Microbial communities attach themselves to microplastics therefore making microplastics a source of waterborne pathogens, which can affect water quality.43
Based on the available data, it is enough to suggest that plastic pollution is major environmental concern for the Great Lakes. If escalating plastic production and environmentally unfriendly waste practices continue, the Great Lakes ecosystem could be in a dire situation. Upon comparing the average concentration of microplastic debris in Lake Superior, Huron and Erie alone, it rivals the known areas of litter accumulation in the oceanic gyres.44 There are multiple entryways into combating the issues of plastic pollution in Chicago’s Lake Michigan. This rest of the unit will go on to explore various opportunities for innovation addressing plastic waste through engineering.
Future of Plastic
Currently, one of the issues at the forefront of plastic waste is its resistance to complete degradation and the accumulation of microplastics in the environment. Three quarters of plastic waste is placed into landfills and the environment each year.45 We are now seeing novel environmental and toxicological issues, like the Great Pacific Garbage Patch and ingestion of microplastics in aquatic species. Just like we are only now learning the long term challenges of plastic production, use and waste, scientists and health professionals are still learning about the effect of microplastics on life and the environment.
These challenges have raised the question on the future of plastic. Bioplastics are becoming a more widely-proposed alternative to traditional petroleum-based plastics. The term bioplastics might be insufficient in describing more sustainable alternatives to petroleum-based plastic, though. Instead, it is suggested the terms bio-based polymer and biodegradeable polymer replace bioplastic for use in the discussion of sustainable alternatives. Bio-based polymers are synthesized with renewable raw materials, but many are still commonly not biodegradable. Biodegradable polymers are able to degrade completely to carbon dioxide when exposed to microorganisms and oxygen (aerobic) processes. Bioplastics can also be degraded anaerobically (absence of oxygen). Some bio-based polymers are degradable (e.g., polylactic acid and polyhydroxyalkanoate) but not all (e.g., biopolyhethylene). Additionally, biodegradable polymers are not always bio-based (e.g., polycaprolactone)46. There are two types of bioplastics that are biodegradable: polyactic acid (PLA) and polyhydroxyalkanoate (PHA).
PLA is a thermoplasic created through bacterial fermentation resulting in lactic acid. The resulting lactic acid is polymerized. Since scientists are able to produce lactic acid relatively inexpensively, it is cheaper to produce than PHA. The cons to PLA is that it is brittle, thermally unstable, and hydrophobic. However, like all polymers, the properties can be altered by its chemical structure or blending with renewable polymers.47
In a nutrient-deficient environment, many bacteria create PHA as food and energy reserves stored in the cytoplasm. PHAs are all dissovable in carbon dioxide and water regardless of their physical properties. Again, like other polymers, properties of PHA can be altered by its chemical structure. Drawbacks to PHA are their high production costs, low yields, and low availability. A solution to the high production cost requires a blend of PHA with renewable resources like starch or cellulose. Blends actually degrade better than PHAs without renewable materials48. Additionally, in a study completed at Ohio State University, scientists claimed to have found a viable alternative to petroleum-based plastic in a blend between organic rubber and Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV). “The new PHBV/NR material has mechanical properties (strength of 28 MPa and toughness of 28 J m−1) and processing windows comparable to those of some commercial plastics, such as PP and HDPE, and can replace some petroleum-based conventional thermoplastics in cast sheets and thermoforms, including those used in food packaging.”49
To truly begin combating plastic waste, it’s not simply enough to engineer bio-based, biodegradable plastics. A 2011 study from the University of Pittsburgh compared biopolymers to petroleum-based plastics and determined that the biopolymers ranked high in terms of their green design, but they also had much larger negative effects on environmental impact from production. “As shown through the LCA results, biopolymers represent decreases in fossil fuel use and global warming potential and increases in other impact categories such as eutrophication, human health impacts, and eco-toxicity. These impacts result both from fertilizer use, pesticide use, and land use change required for agriculture production as well as from the fermentation and other chemical processing steps.”50 Furthermore, in 2017, another study determined that switching to renewable energy sources to produce traditional plastics could reduce greenhouse gas emissions from plastic manufacturing by 50-75%. This is more of a decrease in harmful emissions than if we only switched from traditional plastic production to corn-based PLA, which would only result in a 25% reduction.51 Studies like these lead to a vision of multiple strategies to mitigate plastic pollution. It seems that a combination of different strategies might be the most beneficial in future plastic production and use. Using renewable energy sources in the production of plastics, legislation like the Microbead-Free Water Act of 2015, other newly instituted bans on single-use plastics, continuing engineering of bio-based and biodegradable plastics as well as alternatives, reducing dependence, and repurposing plastic are all valid in the fight against plastic pollution.
Overview of Engineering Design Process
Engineering is a process that can be defined loosely into seven stages. Engineering can be iterative. The process begins with asking a question or defining a problem. By identifying the question or problem, a list of criteria can be determined. Criteria will work as a set of goals for your design. In addition, you must develop your constraints, or factors that will limit your design. Once the criteria and constraints are developed, the research phase begins. Engineers research the problem and even use current technologies and products to influence their ideas. After extensive research, engineers brainstorm possible solutions all the while keeping their criteria and constraints in mind. By using the criteria and constraints, engineers can narrow down their possible solutions and choose the plan they will put into action. Engineers will then create a prototype of the selected plan and will then test that prototype based on the criteria of the design. After evaluating the results, engineers improve upon their design and redesign. This is a cyclic process and is repeated over and over until a desirable result is achieved.52 It is important to note that this design process could be applied to multiple stages of plastic manufacturing and disposal, and it can apply to any product not just plastics.
Teaching Strategies
Engineering Teams
Each year I receive a diverse population of academic abilities in my classroom. In order to meet the needs of all students, I utilize a variety of different strategies to address different learning styles and academic needs. Students work in partners or small groups throughout most of the activities as an engineering team. My students enjoy working in groups and this gives them an opportunity to interact with their peers. I find that this also allows students to support each other in their learning.
Inquiry-based learning and Formative Assessment
I use inquiry-based learning over the entire school year for both science and engineering lessons. In an engineering unit, I use a Driving Question Board (DQB) as we investigate the science behind the engineering problem. Each lesson is framed with an investigative question that students should be able to answer by the end of their investigation. One lesson could span multiple days, so students may need more than one day to develop an appropriate response to the investigation question posed. At the beginning of the school year, I typically develop most or all of the investgative questions. As students gain experience with the routines and procedure of utilizing the DQB, I gradually involve them in the process of creating the questions as we move throughout the year. This requires introducing the engineering problem and asking students to generate questions they have, which is the same process as what I use in science units when I introduce a phenomenon. Before distributing individual DQBs for students to record their conclusions, I try to group questions that build off each other or order them in a way that makes sense for investigation. Students track their conclusions for the questions on their own individual paper tracker, which we keep in the classroom and students refer to each day. I am also able to use students’ conclusions as a formative assessment to determine if they mastered the material for each day.
Investigations
Investigations that students take part in to learn the science behind the engineering include hands-on labs, simulations, informational texts and general research. The various activities allow for a mix of digital and non-digital instruction. My students are typically most engaged in the hands-on labs because they do not always have many kinesthetic opportunities in their other core classes. My students are least engaged with informational texts. To overcome their lack of interest, I try to provide relevant sources and information pertinent to their own lives or anything to do with their local surroundings. Sometimes, like in this unit, I introduce the overall engineering problem with a local connection. I find that relating the content to my students in some way always engages my students more so than something that is not applicable. This also gives students the opportunity to use their own background knowledge and apply it to whatever they are learning.
Summative Assessment
As part of the wrap up to the unit, students partake in an Engineering Summit. During the summit, students present their knowledge of the problem, designs, results, and final conclusion or future plans. For the most part, students generate the criteria for grading. The criteria they suggest and agree upon becomes the rubric for grading their presentations. I facilitate the discussion to ensure that all relevant criteria to show student growth and mastery is included in their decision. In addition to their presentation, students also submit a final report highlighting the components of their presentation and include a reflection of their entire process they underwent in their design.
Classroom Activities
History 101
To launch the unit in an engaging way, students will view Netflix’s History 101 Plastics episode. This documentary will provide a brief history of plastics and the various innovations and uses over the years. The information presented in the documentary does not focus much on the science of plastics, which leaves plenty of opportunity for inquiry. During the video, students will fill out a worksheet documenting the pros and cons of plastics, as well as the challenges mentioned in the video. After the video they will review with their table groups. Students will generate questions they have based on the information provided in the documentary. They will also document pros and cons to plastics, and they will identify plastic disposal as one of the main challenges. Students should be able to generate this information from watching the documentary, however the teacher can provide additional guided questions to research if necessary. This lesson is just an introduction to the rest of the unit, so it is not important for students to dive into the details behind plastic production and waste.
Polymer Models
To address the chemistry of plastics, students will perform two hands on activities. The first activity will be to create models of polymers using paper clips. Students will be introduced to the three types of polymer structures found in plastics: linear, branched, and crosslinked through a reading. Students will then use paper clips to model each type of structure. Students will analyze the structures to identify characteristics of the structures. The second activity will be a hands on lab creating two different types of putty. The lab experience will reinforce the different structural bonds and will allow students to create two different putties to compare different degrees of crosslinking in the polymers created. Students will document their observations of both types of putty and will be able to conclude that higher crosslinking results in a stronger structure.
Life Cycle
Students will be introduced to the concept of a product’s life cycle through a TED-Ed talk titled, “What really happens to the plastic you throw away”. The link to the video is found in the Teacher Resources section below. Students should be able to identify the various stages of the life cycle after watching the video. Students will then form groups and choose to research any of the stages more in depth and create presentations for the whole class. Depending on the grade level and comfortability with research, sources could be provided or student-generated. The research should be used as a way to show students the diverse environmental impacts of a product are not only in the disposal stage. While the rest of this unit is designed to address the end-of-life challenges associated with plastics, all stages offer opportunities for engineering and design. This type of analysis of a product’s life cycle can also be applied to any product.
Plastics Identification
Students will work in lab groups to investigate plastic samples. Students should be given six samples of unidentified plastic. The samples should include one sample of each: polyvinyl chlroride, polystyrene, polyethylene terephthalate, high density polyethylene, low density polyethylene, and polypropylene. Students will run tests on the various plastics to separate them by characteristics. The tests will inlude a density test in water, copper wire test, density in ispropyl alcohol test, reaction to acetone test, oil test and heat test. Students will test the various plastic samples and begin to sort them based on the results they get from the tests. Students will conclude there are different types of plastics with different characteristics.
Plastics Stations
To continue investigating plastics, students will participate in a stations activity. There are seven major plastics groups, and thus I create seven stations. One station for each type of plastic. I typically provide a couple different examples of each type of plastic for each station. You could also ask students and families to collect plastic items and have students sort them into the stations before doing the activity. Most plastics will have a number within the triangular recycle symbol. This is called the resin identification code. Plastics with a 1 would go to Station 1, those with a 2 go to Station 2, and so on. At each station I provide a key facts card that lists the scientific name, abbreviation, common uses, and recyclability. Students take notes at each station and also list characteristics they observe.
Engineering Bioplastics
Students will begin their engineering project for bio-based, biodegradable plastics by learning about the Engineering Design Cycle. Following the steps of the cycle, students will define the problem of petroleum based plastics and define the criteria and constraints for their bioplastic. For this unit, one non-negotiable criteria I will give to all students is that their plastic must be more degradable in comparison to petroleum plastics. In engineering teams, they will research current biodegradable plastics. Then, teams will have the opportunity to analzye the available materials. The materials they choose from are two different plasticizers, various starches, and various additives. Once they investigate the materials, they develop their own formula and mix all their chosen ingredients in a beaker on a hot plate. When ready, they pour their mixture into a mold and allow it to set. Students then observe their molds the next day. Based on the results, they may move into testing the bioplastic or they may revise their formula to create a new mold if their first try is unsuccessful. Once teams achieve a testable mold, students perform various tests depending on their chosen criteria and analyze the results of their testing. The tests should include degradability tests by burying the biodegradable plastics to see if they degrade over time compared to synthetic plastic. This may be analyzed over time to achieve usable data. Other tests can be performed before this test to make an initial conclusion and analysis of the bioplastic. Teachers may determine how many iterative designs they will allow students to run through depending on time and materials. The project concludes with a gallery walk of the different products from each team or class presentations. The format is selected by the students.
Adopt-A-Beach
As a culminating project for this unit, I will coordinate my class to participate in the Adopt-A-Beach proram. This is a program coordinated by the Alliance for the Great Lakes. I would organize a beach, date, and time for my students to travel to a local Chicago beach to participate in cleaning up the beach. As part of the clean up process, the Alliance asks students to collect litter and keep record of what is collected. The information is then entered by students into their database. The database is accessible for analysis of the historical data of each beach clean up, which can be used for further discussion around plastic pollution. In the classroom, students could also analyze their own data in more depth to determine the type of plastic they collect and the percentage of each type collected. They can then discuss the results to compare if their data matches what research has said about the manufacture and disposal of each type of plastic as well as what percentage is recyclable.
Appendix: Implementing District Standards
Next Generation Science Standards
This unit was designed to meet performance expectations found in the Next Generation Science Standards (NGSS). The standards identified below are suggestions of the science and engineering standards intended to be addressed when this unit was created, however the content of this unit could be relevant to additional standards within NGSS.
Performance Expectations
MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures.
MS-PS1-3. Gather and makes sense of information to describe that synthetic materials come from natural resources and impact society.
MS-ESS3-3. Apply scientific principles to design a method for monitoring and minimizing a human impact on the environment.
MS-ETS1-1. Define the criteria and constraints of a design problem with sufficient precision to ensure a successful solution, taking into account relevant scientific principles and potential impacts on people and the natural environment that may limit possible solutions.
MS-ETS1-2. Define the criteria and constraints of a design problem with sufficient precision to ensure a successful solution, taking into account relevant scientific principles and potential impacts on people and the natural environment that may limit possible solutions.
Disciplinary Core Ideas
PS1.A. Structure and Properties of Matter
Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. (MS-PS1-1)
Solids may be formed from molecules, or they may be extended structures with repeating subunits (e.g., crystals). (MS-PS1-1)
Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. (MS-PS1-3)
PS1.B. Chemical Reactions
Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants. (MS-PS1-3)
ESS2.C. Human Impacts on Earth Systems
Human activities have significantly altered the bioshpere, sometimes damaging or destroying natural habitats and causing the extinction of other species. But changes to Earth’s environments can have different impacts (negative and positive) for different living things.
Typically as human populations and per capita consumption of natural resources increase, so do the negative impacts on Earth unless the activities and technologies involved are engineered otherwise. (MS-ESS3-3)
ETS1.A. Defining and Delimiting Engineering Problems
The more precisely a design task’s criteria and constraints can be defined, the more likely it is that the desgined solution will be successful. Specification of constraints includes consideration of scientific principles and other relevant knowledge that is likely to limit possible solutions. (MS-ETS1-1)
ETS1.B. Developing Possible Solutions
There are systematic processes for evaluating solutions with respect to how well they meet the criteria and constraints of a problem. (MS-ETS1-2)
Science & Engineering Practices
Asking Questions and Defining Problems, Developing and Using Models, Planning and Carrying Out Investigations, Constructing Explanations and Designing Solution, Engaging in Argument form Evidence, and Obtaining, Evaluating, and Communicating Information
Crosscutting Concepts
Scale, Proportion, and Quantity, Systems and System Models, Structure and Function, Influence of Science, Engineering, and Technology on Society and the Natural World
Teacher Resources
https://plastics.americanchemistry.com/default.aspx
This site includes a lot of basic information on plastics.
https://www.middleschoolchemistry.com/lessonplans/
This website includes a lot of resources and lesson ideas regarding chemistry and materials. This could be most helpful when exploring the chemistry of polymers.
https://www.ted.com/talks/emma_bryce_what_really_happens_to_the_plastic_you_throw_away?language=en
This is the TED-Ed talk to introduce the life cycle of a plastic bottle. You will find other resources and lesson ideas in links contained in the content of this website that could also be used or provided as research resources for students.
Bibliography
Babu, Ramesh P, Kevin O'connor, and Ramakrishna Seeram. “Current Progress on Bio-Based Polymers and Their Future Trends.” Progress in Biomaterials 2, no. 1 (March 18, 2013). https://doi.org/10.1186/2194-0517-2-8.
“Bakelite First Synthetic Plastic - National Historic Chemical Landmark.” American Chemical Society. Accessed June 14, 2020. https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/bakelite.html.
“Biodegradable Plastic: Its Promises and Consequences.” Dartmouth Undergraduate Journal of Science, March 2, 2013. https://sites.dartmouth.edu/dujs/2013/03/02/biodegradable-plastic-its-promises-and-consequences/.
Boyd, Jane E. “Celluloid: The Eternal Substitute.” Science History Institute, April 30, 2019. https://www.sciencehistory.org/distillations/celluloid-the-eternal-substitute.
“Chicago Beaches: Guide to Local Beaches on Lake Michigan.” Choose Chicago, September 3, 2019. https://www.choosechicago.com/articles/parks-outdoors/chicagos-beach-guide/.
Cho, Renee. “The Truth About Bioplastics.” State of the Planet. Columbia University, November 20, 2018. https://blogs.ei.columbia.edu/2017/12/13/the-truth-about-bioplastics/.
“Cracking.” Encyclopedia Britannica. Encyclopedia Britannica, inc., June 2, 2020. https://www.britannica.com/technology/cracking-chemical-process.
Driedger, Alexander G.j., Hans H. Dürr, Kristen Mitchell, and Philippe Van Cappellen. “Plastic Debris in the Laurentian Great Lakes: A Review.” Journal of Great Lakes Research 41, no. 1 (2015): 9–19. https://doi.org/10.1016/j.jglr.2014.12.020.
“Engineering Design Process.” Teach Engineering. University of Colorado, Boulder. Accessed June 14, 2020. https://www.teachengineering.org/k12engineering/designprocess.
Freinkel, Susan. “A Brief History of Plastic's Conquest of the World.” Scientific American. Scientific American, May 29, 2011. https://www.scientificamerican.com/article/a-brief-history-of-plastic-world-conquest/.
Gawlowicz, Susan. "Researchers Study Plastic Pollution in Great Lakes." Rochester Institute of Technology. December 19, 2016. Accessed July 08, 2020. https://www.rit.edu/news/researchers-study-plastic-pollution-great-lakes.
Geyer, Roland, Jenna R. Jambeck, and Kara Lavender Law. “Production, Use, and Fate of All Plastics Ever Made.” Science Advances 3, no. 7 (July 19, 2017). https://doi.org/10.1126/sciadv.1700782.
Gilbert, Marianne. “Plastics Materials: Introduction and Historical Development.” Essay. In Brydson's Plastics Materials, 8th ed., 4–8. Oxford, UK: Butterworth-Heinemann, 2017.
Hoffman, Matthew J., and Eric Hittinger. “Inventory and Transport of Plastic Debris in the Laurentian Great Lakes.” Marine Pollution Bulletin 115, no. 1-2 (2017): 273–81. https://doi.org/10.1016/j.marpolbul.2016.11.061.
“How Plastics Are Made.” American Chemistry Council. Accessed July 14, 2020. https://plastics.americanchemistry.com/How-Plastics-Are-Made/.
“Lifecycle of a Plastic Product.” Lifecycle of a Plastic Product. American Chemistry Council. Accessed June 14, 2020. https://plastics.americanchemistry.com/Life-Cycle/.
Loria, Kevin. “How to Eat Less Plastic.” Consumer Reports, April 30, 2020. https://www.consumerreports.org/health-wellness/how-to-eat-less-plastic-microplastics-in-food-water/.
“Microplastics in the Great Lakes: Becoming Benthic.” ScienceDaily. Geological Society of America, September 23, 2019. http://www.sciencedaily.com/releases/2019/09/190923164532.htm.
McGowan, Veronica, Phillip Bell, and Marcia Ventura. “How Can Students' Everyday Experiences Support Science Learning through Engineering Design?” Stem Teaching Tools, November 2016. http://stemteachingtools.org/brief/39.
“Pathways Program Removes 86 Lbs. of Litter from Foster Beach.” Alliance for the Great Lakes, August 17, 2018. https://greatlakes.org/2018/08/pathways-program/.
“Plastics: Material-Specific Data.” EPA. Environmental Protection Agency, October 30, 2019. https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data#PlasticsTableandGraph.
“Polymerization: How Plastic Materials Are Made - Polymerization.” Craftech Industries, November 14, 2019. https://www.craftechind.com/polymerization-how-plastic-materials-are-made/.
SEPUP Issues and Physical Science. Ronkonkoma, NY: Lab-Aids, Inc., 2006.
Tabone, Michaelangelo D., James J. Cregg, Eric J. Beckman, and Amy E. Landis. “Sustainability Metrics: Life Cycle Assessment and Green Design in Polymers.” Environmental Science & Technology 44, no. 21 (2010): 8264–69. https://doi.org/10.1021/es101640n.
“The Basics: Polymer Definition and Properties.” The Basics: Polymer Definition and Properties. American Chemistry Council. Accessed June 14, 2020. https://plastics.americanchemistry.com/plastics/The-Basics/.
“The Microbead-Free Waters Act.” U.S. Food and Drug Administration. FDA. Accessed July 14, 2020. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/microbead-free-waters-act-faqs.
Wagner, Martin, Christian Scherer, Diana Alvarez-Muñoz, Nicole Brennholt, Xavier Bourrain, Sebastian Buchinger, Elke Fries, et al. “Microplastics in Freshwater Ecosystems: What We Know and What We Need to Know.” Environmental Sciences Europe 26, no. 1 (2014). https://doi.org/10.1186/s12302-014-0012-7.
Zhao, Xiaoying, Katrina Cornish, and Yael Vodovotz. “Synergistic Mechanisms Underlie the Peroxide and Coagent Improvement of Natural-Rubber-Toughened Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Mechanical Performance.” MDPI. Multidisciplinary Digital Publishing Institute, March 26, 2019. https://www.mdpi.com/2073-4360/11/3/565/htm.
Endnotes
1 “The Basics: Polymer Definition and Properties,” The Basics: Polymer Definition and Properties (American Chemistry Council), accessed June 14, 2020, https://plastics.americanchemistry.com/plastics/The-Basics/
2 Hannah Ritchie, “Plastic Pollution,” Our World in Data, 2018, https://ourworldindata.org/plastic-pollution.
3 Roland Geyer, Jenna R. Jambeck, and Kara Lavender Law, “Production, Use, and Fate of All Plastics Ever Made,” Science Advances 3, no. 7 (July 19, 2017), https://doi.org/10.1126/sciadv.1700782
4 “Plastics: Material-Specific Data,” EPA (Environmental Protection Agency, October 30, 2019), https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data#PlasticsTableandGraph
5 “Plastics: Material-Specific Data,” EPA (Environmental Protection Agency, October 30, 2019), https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data.
6 Roland Geyer, Jenna R. Jambeck, and Kara Lavender Law, “Production, Use, and Fate of All Plastics Ever Made,” Science Advances 3, no. 7 (July 19, 2017), https://doi.org/10.1126/sciadv.1700782
7 Martin Wagner et al., “Microplastics in Freshwater Ecosystems: What We Know and What We Need to Know,” Environmental Sciences Europe 26, no. 1 (September 2014), https://doi.org/10.1186/s12302-014-0012-7.
8 Veronica McGowan, Phillip Bell, and Marcia Ventura, “How Can Students' Everyday Experiences Support Science Learning through Engineering Design?,” Stem Teaching Tools, November 2016, http://stemteachingtools.org/brief/39
9 Jane E. Boyd, “Celluloid: The Eternal Substitute,” Science History Institute, April 30, 2019, https://www.sciencehistory.org/distillations/celluloid-the-eternal-substitute
10 “Bakelite First Synthetic Plastic - National Historic Chemical Landmark,” American Chemical Society, accessed June 14, 2020, https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/bakelite.html
11 Marianne Gilbert, “Plastics Materials: Introduction and Historical Development,” in Brydson's Plastics Materials, 8th ed. (Oxford, UK: Butterworth-Heinemann, 2017), pp. 4-8.
12 Susan Freinkel, “A Brief History of Plastic's Conquest of the World,” Scientific American (Scientific American, May 29, 2011), https://www.scientificamerican.com/article/a-brief-history-of-plastic-world-conquest/.
13 Marianne Gilbert, “Plastics Materials: Introduction and Historical Development,” in Brydson's Plastics Materials, 8th ed. (Oxford, UK: Butterworth-Heinemann, 2017), pp. 4-8.
14 “Plastics: Material-Specific Data,” EPA (Environmental Protection Agency, October 30, 2019), https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data.
15 “How Plastics Are Made,” How Plastics Are Made (American Chemistry Council), accessed July 14, 2020, https://plastics.americanchemistry.com/How-Plastics-Are-Made/.
16 “How Plastics Are Made,” How Plastics Are Made (American Chemistry Council), accessed July 14, 2020, https://plastics.americanchemistry.com/How-Plastics-Are-Made/.
17 SEPUP Issues and Physical Science (Ronkonkoma, NY: Lab-Aids, Inc., 2006).
18 “Cracking,” Encyclopædia Britannica (Encyclopædia Britannica, inc., June 2, 2020), https://www.britannica.com/technology/cracking-chemical-process.
19 “Lifecycle of a Plastic Product.” Lifecycle of a Plastic Product. American Chemistry Council. Accessed June 14, 2020. https://plastics.americanchemistry.com/Life-Cycle/.
20 “Polymerization: How Plastic Materials Are Made - Polymerization,” Craftech Industries, November 14, 2019, https://www.craftechind.com/polymerization-how-plastic-materials-are-made/
21 “Polymerization: How Plastic Materials Are Made - Polymerization,” Craftech Industries, November 14, 2019, https://www.craftechind.com/polymerization-how-plastic-materials-are-made/
22 “Lifecycle of a Plastic Product.” Lifecycle of a Plastic Product. American Chemistry Council. Accessed June 14, 2020. https://plastics.americanchemistry.com/Life-Cycle/.
23 “Lifecycle of a Plastic Product.” Lifecycle of a Plastic Product. American Chemistry Council. Accessed June 14, 2020. https://plastics.americanchemistry.com/Life-Cycle/.
24 “Lifecycle of a Plastic Product.” Lifecycle of a Plastic Product. American Chemistry Council. Accessed June 14, 2020. https://plastics.americanchemistry.com/Life-Cycle/.
25 Hannah Ritchie, “FAQs on Plastic,” Our World in Data, 2018, https://ourworldindata.org/faq-on-plastics.
26 Hannah Ritchie, “FAQs on Plastic,” Our World in Data, 2018, https://ourworldindata.org/faq-on-plastics.
27 “Lifecycle of a Plastic Product.” Lifecycle of a Plastic Product. American Chemistry Council. Accessed June 14, 2020. https://plastics.americanchemistry.com/Life-Cycle/.
28 “Lifecycle of a Plastic Product.” Lifecycle of a Plastic Product. American Chemistry Council. Accessed June 14, 2020. https://plastics.americanchemistry.com/Life-Cycle/.
29 Susan Gawlowicz, "Researchers Study Plastic Pollution in Great Lakes," Rochester Institute of Technology, December 19, 2016, accessed July 08, 2020, https://www.rit.edu/news/researchers-study-plastic-pollution-great-lakes
30 Matthew J. Hoffman and Eric Hittinger, “Inventory and Transport of Plastic Debris in the Laurentian Great Lakes,” Marine Pollution Bulletin 115, no. 1-2 (2017): pp. 273-281, https://doi.org/10.1016/j.marpolbul.2016.11.061.
31 Alexander G.j. Driedger et al., “Plastic Debris in the Laurentian Great Lakes: A Review,” Journal of Great Lakes Research 41, no. 1 (2015): pp. 9-19, https://doi.org/10.1016/j.jglr.2014.12.020.
32 Alexander G.j. Driedger et al., “Plastic Debris in the Laurentian Great Lakes: A Review,” Journal of Great Lakes Research 41, no. 1 (2015): pp. 9-19, https://doi.org/10.1016/j.jglr.2014.12.020.
33 “Chicago Beaches: Guide to Local Beaches on Lake Michigan,” Choose Chicago, September 3, 2019, https://www.choosechicago.com/articles/parks-outdoors/chicagos-beach-guide/.
34 “Pathways Program Removes 86 Lbs. of Litter from Foster Beach,” Alliance for the Great Lakes, August 17, 2018, https://greatlakes.org/2018/08/pathways-program/.
35 Alexander G.j. Driedger et al., “Plastic Debris in the Laurentian Great Lakes: A Review,” Journal of Great Lakes Research 41, no. 1 (2015): pp. 9-19, https://doi.org/10.1016/j.jglr.2014.12.020.
36 Alexander G.j. Driedger et al., “Plastic Debris in the Laurentian Great Lakes: A Review,” Journal of Great Lakes Research 41, no. 1 (2015): pp. 9-19, https://doi.org/10.1016/j.jglr.2014.12.020.
37 Alexander G.j. Driedger et al., “Plastic Debris in the Laurentian Great Lakes: A Review,” Journal of Great Lakes Research 41, no. 1 (2015): pp. 9-19, https://doi.org/10.1016/j.jglr.2014.12.020.
38 “Microplastics in the Great Lakes: Becoming Benthic,” ScienceDaily (Geological Society of America, September 23, 2019), http://www.sciencedaily.com/releases/2019/09/190923164532.htm.
39 Alexander G.j. Driedger et al., “Plastic Debris in the Laurentian Great Lakes: A Review,” Journal of Great Lakes Research 41, no. 1 (2015): pp. 9-19, https://doi.org/10.1016/j.jglr.2014.12.020.
40 “The Microbead-Free Waters Act,” U.S. Food and Drug Administration (FDA), accessed July 14, 2020, https://www.fda.gov/cosmetics/cosmetics-laws-regulations/microbead-free-waters-act-faqs.
41 Alexander G.j. Driedger et al., “Plastic Debris in the Laurentian Great Lakes: A Review,” Journal of Great Lakes Research 41, no. 1 (2015): pp. 9-19, https://doi.org/10.1016/j.jglr.2014.12.020.
42 Alexander G.j. Driedger et al., “Plastic Debris in the Laurentian Great Lakes: A Review,” Journal of Great Lakes Research 41, no. 1 (2015): pp. 9-19, https://doi.org/10.1016/j.jglr.2014.12.020.
43 Martin Wagner et al., “Microplastics in Freshwater Ecosystems: What We Know and What We Need to Know,” Environmental Sciences Europe 26, no. 1 (September 2014), https://doi.org/10.1186/s12302-014-0012-7.
44 Alexander G.j. Driedger et al., “Plastic Debris in the Laurentian Great Lakes: A Review,” Journal of Great Lakes Research 41, no. 1 (2015): pp. 9-19, https://doi.org/10.1016/j.jglr.2014.12.020.
45 “Plastics: Material-Specific Data,” EPA (Environmental Protection Agency, October 30, 2019), https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data#PlasticsTableandGraph
46 Ramesh P Babu, Kevin O'connor, and Ramakrishna Seeram, “Current Progress on Bio-Based Polymers and Their Future Trends,” Progress in Biomaterials 2, no. 1 (March 18, 2013), https://doi.org/10.1186/2194-0517-2-8.
47 “Biodegradable Plastic: Its Promises and Consequences,” Dartmouth Undergraduate Journal of Science, March 2, 2013, https://sites.dartmouth.edu/dujs/2013/03/02/biodegradable-plastic-its-promises-and-consequences/.
48 “Biodegradable Plastic: Its Promises and Consequences,” Dartmouth Undergraduate Journal of Science, March 2, 2013, https://sites.dartmouth.edu/dujs/2013/03/02/biodegradable-plastic-its-promises-and-consequences/.
49 Xiaoying Zhao, Katrina Cornish, and Yael Vodovotz, “Synergistic Mechanisms Underlie the Peroxide and Coagent Improvement of Natural-Rubber-Toughened Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Mechanical Performance,” MDPI (Multidisciplinary Digital Publishing Institute, March 26, 2019), https://www.mdpi.com/2073-4360/11/3/565/htm.
50 Michaelangelo D. Tabone et al., “Sustainability Metrics: Life Cycle Assessment and Green Design in Polymers,” Environmental Science & Technology 44, no. 21 (2010): pp. 8264-8269, https://doi.org/10.1021/es101640n.
51 Renee Cho, “The Truth About Bioplastics,” State of the Planet (Columbia University, November 20, 2018), https://blogs.ei.columbia.edu/2017/12/13/the-truth-about-bioplastics/
52 “Engineering Design Process,” Teach Engineering (University of Colorado, Boulder), accessed June 14, 2020, https://www.teachengineering.org/k12engineering/designprocess
Comments (0)
THANK YOU — your feedback is very important to us! Give Feedback
