Blue Balloons indicate where water samples will be taken for students to conduct comparative tests including pH and lead tests.
Hunters Point Naval Shipyard (HPS)

The purpose of this unit for me is to motivate students to practice thinking scientifically about things they already care deeply about: their safety in their homes and communities in relation to knowledge they gain about elements and compounds. The topic for this unit will be the historic use and current status of the Hunters Point Naval Station in San Francisco and its ongoing effects on local air and water quality, as well as its impact on potential future use of the area. My goal in bringing this topic to my classroom is to relate it to our study of characteristic properties of elements and compounds to events and conditions in the world, specifically my students' world. To understand and interact safely with any urban land area, its historic uses should be known. The fact that those historic uses involve toxins and may very likely be negatively impacting the health of many people, should be known by not only the adjacent neighborhood, everyone in the San Francisco community has the responsibility to become aware of such hazards.
As a science teacher it is important to me that the content presented to students not be reduced to a list of "facts" for them to read about and attempt to memorize. Science is by its nature engaging with the world. My interest in this topic stems from wanting my students to care about their environment, to want to know more about it, and to feel empowered by that knowledge. While studying the history of the Hunters Point Naval Station, student will be focused on: the presence of various toxins that have accumulated on the site, primarily since World War II. The activities in the unit are designed to guide students learning in the way in which those toxins came to be there, how they interact with the physical environment, they ways in which they may come into contact with the living organisms in the area, what the negative impact thtat contact has or may have on the various organisms both individually and systematically, and what can be done to stem and hopefully reverse the damage.
The unit is designed to take 3 - 4 weeks. Throughout the unit students will build the vocabulary necessary to speak and write scientifically about this topic. Some vocabulary will be given directly and explicitly to all students, other vocabulary will come as a result of each student's specific research, questioning, and experimentation. Students will record, define, and reference new vocabulary terms in their science notebooks. The first and second week of the unit students will complete a module on chemicals and the environment, through various activities, readings, and videos the module introduces the students to chemistry, toxicology, routes of exposure, dose response, risk assessment, and risk reduction/elimination.
Students will apply the concepts covered in the generalized content module to chemical conditions that have developed at Hunters Point Naval Shipyard. The students will conduct computer research looking at sets of health surveillance data for different time periods and different communities. They will look at data for San Francisco neighborhoods, neighborhoods in other communities where a Superfund Site has been identified, as well as communities that have no such site. Students will work in teams to identify and question any trends they observe in the data, and list any inferences their team makes about those trends. Students will read about the different toxins found at the Hunters Point Naval Station. They will focus their studies on three potential hazards: the presence of asbestos, lead, and mercury throughout the site. For each, students will examine the substance's effects on people, how they're exposed, time and level of exposure related to ill health effects, and what's being done to clean up the shipyard and protect the Hunters Point neighborhood, San Francisco's citizens, from exposure. Students will compare water samples from ten locations along the city's bayside coast, collected throughout the fall semester. They will measure at least pH and lead content levels.
Geographically, San Francisco is a small town (only 49 square miles), but with a diverse population of around 750,000 it is as culturally and economically urban as larger cities throughout the country. Like other multi-ethnic communities, San Francisco faces issues of racial imbalance within our neighborhoods and schools. For several of the students in my classes, their home neighborhood borders the Hunters Point Naval Station. The Bayview Hunters Point neighborhood is predominantly African American; it is also disproportionately hard hit by many illnesses, including asthma, respiratory illness, and breast cancer. The majority of my students may not even be aware of the existence of the site and the health implications its presence has for their classmates. Health surveys show that in Bayview Hunters Point, rates of cervical and breast cancer were found to be double the rate found in other parts of the Bay Area, and hospitalization rates for congestive heart failure, hypertension, diabetes, and emphysema were found to be more than three times the statewide average. These illnesses are strongly associated with environmental factors affecting air and water quality. Bayview Hunters Point and the bordering neighborhood of Potrero Hill account for more than half of all infant mortality in the San Francisco area; one study found that the overall rate of birth defects for the area was 44.3 per 1000 births, compared with 33.1 per 1000 births for the rest of San Francisco County. Through guest speakers, teacher presentation, and assigned reading, students will learn about the history of the Hunters Point Naval Station, it's designation as a Super Fund site, and the identification of the specific toxins which resulted in that designation.
According to the Environmental Protection Agency (EPA) website (http://www.epa.gov), it is their responsibility to identify the most serious health risks to the peoples of the US and its territories. Superfund is the name given to the environmental program established to address abandoned hazardous waste sites. It is also the name of the fund established by the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended (CERCLA statute, CERCLA overview). This law was enacted in the wake of the discovery of toxic waste dumps such as Love Canal and Times Beach in the 1970s. It allows the EPA to clean up such sites and to compel responsible parties to perform cleanups or reimburse the government for EPA-lead cleanups. The NPL is the list of national priorities among the known releases or threatened releases of hazardous substances, pollutants, or contaminants throughout the United States and its territories. The NPL is intended primarily to guide the EPA in determining which sites warrant further investigation. The Hunters Point Naval Shipyard's Superfund site status qualifies it for the National Priorities List. "Responsible parties" include not only privately-owned businesses, many Superfund sites are the result of hazards created by activities of the government, as is the case with the Hunters Point Naval Shipyard and many other former military sites.
Hunters Point was established as a commercial shipyard in 1870. In 1939, the Navy purchased its 47 acres and in 1942, seized additional land from the neighborhood, evicting 100 families from 86 homes and closing 23 businesses. They were told to be prepared to evacuate within 48 hours, although they may have had two weeks. Between 1935 and 1975, the land area of the HPS facility grew by more than 300%, from less than 100 acres to the approximately 420 acres that it consists of today. According to a 1997 study (Tetra Tech, Uribe & Associates, and LFR 1997) sited in the Feasibility Study Report for Parcel F, prepared under: Naval Facilities Engineering Command Contract Number N68711-03-D-5106 Contract Task Order 004, the shipyard contains three types of artificial fill:
- serpentine bedrock-derived fill, primarily serpentine with chert, shale, and related materials;
- industrial fill (including sandblast waste 1, construction debris, and dredged material); and
- backfill consisting of poorly graded sands and gravel.
There were 18,000 employed at the shipyard in 1945. In 1946, the National Radiological Defense Laboratory was formed and given the task of overseeing the decontamination and disposition of ships that participated in the nuclear weapons testing in the Bikini Atoll. The NRDL was also assigned to study the effects of nuclear weapons and to develop counter measures to those effects. Hunters Point Naval Shipyard buildings were used for radioactive laboratory operations, cyclotron operations, animal studies, and material storage and/or processing.
Ship berths (piers) are known locations of decontamination operations and residues from these operations were potentially disposed of at the shipyard or discharged into the sanitary and storm drain system. Building 365 was used as a decontamination center for personnel working in Building 364 and participating in the hot barge work. The radionuclides associated with the decontamination activities are plutonium-239, cesium-137, and strontium-90. 2
Following the war, employment on the Station dropped to ~8,500 civilians (I'm unsure of the number of military personnel at this point). HP Naval Shipyard was decommissioned in 1974. For the next ten years, the Navy leased the land to a private contractor who operated a ship repair business and sublet to other small businesses. Toward the end of that period, 1985, there was a political battle waged in the City over the homeporting of the USS Missouri. In 1986, the SF District Attorney investigated 20 on-site areas for improper waste disposal practices by Triple A Machine Shop, Inc., the Navy's lessee. Triple A Machine Shop was ultimately indicted and convicted for illegal disposal of hazardous substances. In 1989, the Shipyard was added to the National Priorities List as a Superfund site and in 1991 was permanently closed.
Since it's operational closing, several incidents have occurred on the base, affecting the residents of the Bayview/Hunter's Point community. In August of 2000 a fire burned for three weeks before even being acknowledged to the community, after there were many complaints of respiratory health problems reported, complaints made about smoke coming from the base, etc. According to the ATSDR, approximately 37% of the landfill area burned over the course of three months. No readings were taken during the immediate time of the fire. The ATSDR concluded that no toxins that would cause long-term, permanent health conditions occurred based on later readings and soil samples taken after the fire. In August of 2002, migrating methane gas was detected in the nearby neighborhood. It is known that the land contains covered areas of asbestos and, since leveling-off the redevelopment area began there have been numerous complaints of respiratory problems, headaches, etc. from residents, teachers, business owners in the area.
On January 22, 1992, EPA signed a Federal Facilities Agreement (FFA) with the Navy and the State of California to better coordinate the environmental investigation and cleanup. To expedite investigation and cleanup, the site was divided into 6 parcels, A through F. Parcel F is the offshore parcel. To date, the Navy has completed a significant amount of fieldwork for Parcels A through E. Additional offshore sampling was conducted for Parcel F, and the shipyard's shoreline areas which might impact Parcel F, in 2002 and 2003. Data gaps sampling on Parcel E, groundwater sampling throughout the Shipyard, various treatability studies, and removal actions are ongoing. In 2004 the Navy proposed to subdivide Parcel E into parts— E and E2. Parcel E2 contains the landfill. 3
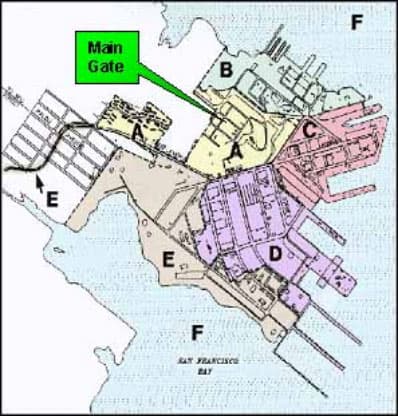
Subdivisions of Hunters Point Naval Shipyard. Parcel A was released by the Navy to San Francisco in 2004. Parcel F is the aquatic division of the shipyard.
In 2004, the Naval Facilities Engineering Command issued a report on the historic exposure of the site to radioactive materials and the current status of the assessment and cleanup. There were 90 sites identified, broken down into six categories. "Scoping survey" sites have yet to be examined for the presence of radioactive materials. According to the report, there were still 34 Scoping Survey sites. So, at 60% of the total identified sites still need to be evaluated for the presence of radioactive material. "Characterization Survey" refers to sites where radioactivity has been identified, but the amount and specific type have not; there were 20 such sites. 5 Sites are classified as "Cleanup Required." A single site requires a "Final Status Survey," which means that it needs to be resurveyed for possible release. 28 of the sites are up for "Review Final Status Survey Report," where the final survey has been conducted but not yet acted upon. And finally, two sites require "No Further Action." 4
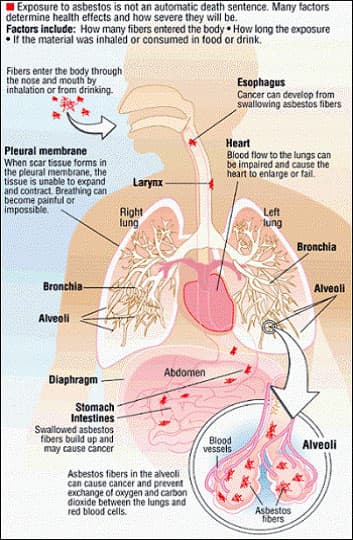
When a substance is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. This release does not always lead to exposure. You are exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking the substance, or by skin contact. Substances differ greatly in toxicity, route of exposure, and dose response. So, a substance that is toxic may be much more hazardous via one route of exposure versus another; for example, elemental mercury that is swallowed does not present the same level of hazard as elemental mercury vapors that are inhaled. Following is an examination of asbestos and mercury, two of the toxins present at Hunters Point Naval shipyard that the students will be learning about.
According to the Toxicological Profile for Asbestos, for the Department of Health and Human Services, if exposed to asbestos, many factors determine whether one will be harmed. These factors include the dose (how much), the duration (how long), the fiber type (mineral form and size distribution), and the manner of contact with it. One must also consider the other chemicals exposed to and as well as age, sex, diet, family traits, lifestyle (including smoking tobacco), and state of health. 5
Asbestos is the name given to a group of six different fibrous minerals (amosite, chrysotile, crocidolite, and the fibrous varieties of tremolite, actinolite, and anthophyllite) that occur naturally in the environment. One of these, namely chrysotile, belongs to the serpentine family of minerals, while all of the others belong to the amphibole family. All forms of asbestos are hazardous, and all can cause cancer, but amphibole forms of asbestos are considered to be somewhat more hazardous to health than chrysotile. Asbestos minerals consist of thin, separable fibers that have a parallel arrangement. Nonfibrous forms of tremolite, actinolite, and anthophyllite also are found naturally. However, because they are not fibrous, they are not classified as asbestos minerals. Amphibole asbestos fibers are generally brittle and often have a rod- or needle-like shape, whereas chrysotile asbestos fibers are flexible and curved. Chrysotile, also known as white asbestos, is the predominant commercial form of asbestos; amphiboles are of minor commercial importance. Asbestos fibers do not have any detectable odor or taste. They do not dissolve in water or evaporate and are resistant to heat, fire, chemical and biological degradation. Because of these properties, asbestos has been mined for use in a wide range of manufactured products, mostly in building materials, friction products, and heat-resistant fabrics. Since asbestos fibers may cause harmful health effects in people who are exposed, all new uses of asbestos have been banned in the United States by the EPA. 6
Asbestos is present, or suspected to be present at subdivisions B, C, D, and E of the Hunters Point Shipyard. Section A is no longer part of the Superfund site, having been released to the city of San Francisco; but, as mentioned above, there is still a great deal of community concern about the presence of asbestos on Parcel A where the monitoring has been inconsistent. The concerns on Parcel F are the presence of radionuclides, PCBs, and metals (copper, lead, and mercury). The following sections address asbestos and mercury.
You are most likely to be exposed to asbestos by breathing in asbestos fibers that are suspended in air. These fibers can come from naturally occurring sources of asbestos or from the wearing down or disturbance of manufactured products including insulation, automotive brakes and clutches, ceiling and floor tiles, dry wall, roof shingles, and cement…However, these products do not always contain asbestos. Low levels of asbestos that present little, if any, risk to your health can be detected in almost any air sample You can also be exposed to asbestos by drinking asbestos fibers that are present in water. Even though asbestos does not dissolve in water, fibers can enter water by being eroded from natural deposits or piles of waste asbestos, from asbestos-containing cement pipes used to carry drinking water, or from filtering through asbestos-containing filters. 7
The mesothelium is a membrane that covers and protects most of the internal organs of the body. It is composed of two layers of cells: One layer immediately surrounds the organ; the other forms a sac around it. The mesothelium produces a lubricating fluid that is released between these layers, allowing moving organs (such as the beating heart and the expanding and contracting lungs) to glide easily against adjacent structures. 8
Asbestos workers have increased chances of getting two principal types of cancer: cancer of the lung tissue itself and mesothelioma, a cancer of the thin membrane that surrounds the lung and other internal organs. These diseases do not develop immediately following exposure to asbestos, but appear only after a number of years. There is also some evidence from studies of workers that breathing asbestos can increase the chances of getting cancer in other locations (for example, the stomach, intestines, esophagus, pancreas, and kidneys), but this is less certain. Members of the public who are exposed to lower levels of asbestos may also have increased chances of getting cancer, but the risks are usually small and are difficult to measure directly. Lung cancer is usually fatal, while mesothelioma is almost always fatal, often within a few months of diagnosis. 9 Mesothelioma is the rarest of the asbestos related diseases, however, the incidence rate has increased over the past 20 years. Currently about 2,000 new cases are diagnosed each year (Mesothelioma: Questions and Answers, 2002). Research into the disease continues. In 2007 the U.S. Food and Drug Administration approved a blood test, known as Mesomark, to help with the early diagnosis of mesothelioma (CDRH Consumer Information, 2007).
Treatment for mesothelioma depends on the location of the cancer, the stage of the disease, and the patient's age and general health. Standard treatment options include surgery, radiation therapy, and chemotherapy. Sometimes, these treatments are combined…To relieve symptoms and control pain, the doctor may use a needle or a thin tube to drain fluid that has built up in the chest or abdomen. The procedure for removing fluid from the chest is called thoracentesis. Removal of fluid from the abdomen is called paracentesis. Drugs may be given through a tube in the chest to prevent more fluid from accumulating. Radiation therapy and surgery may also be helpful in relieving symptoms. 10
The levels of asbestos in air that lead to lung disease depend on several factors. The most important of these are (1) how long you were exposed, (2) how long it has been since your exposure started, and (3) whether you smoked cigarettes. Cigarette smoking and asbestos exposure increase your chances of getting lung cancer. 11 Because of the high correlation between exposure, smoking, and lung cancer, I will have students examine and compare healthy lung tissue to diseased lung tissue as one of their lab activities, possibly having them create their own model for the combined effects.
Asbestos exposure in both children and adults may occur while breathing air in or near buildings (public or private) containing asbestos building materials or near asbestos-related industrial operations. Children breathe differently and have different lung structures than adults. It is not known if these differences may cause a greater amount of asbestos fibers to stay in the lungs of a child when they are breathed in than in the lungs of an adult. Children drink more fluids per kilogram of body weight than adults and can also be exposed through asbestos-contaminated drinking water. Eating asbestos-contaminated soil and dust is another source of exposure for children. Certain children intentionally eat soil, and all young children eat more soil than adults through hand-to-mouth activities. Historically, family members have also been exposed to asbestos that was carried home on the clothing of other family members who worked in asbestos mines or mills. Breathing of asbestos fibers may result in difficulty in breathing, lung cancer, or mesothelioma (another form of cancer associated with asbestos exposure). These diseases usually appear many years following the first exposure to asbestos and are therefore not likely to be seen in children. But since it may take up to 40 or more years for the effects of exposure to be seen, people who have been exposed to asbestos at a young age may be more likely to contract these diseases than those who are first exposed later in life. In the small number of studies that have specifically looked at asbestos exposure in children, there is no indication that younger people might develop asbestos-related diseases more quickly than older people. Developing fetuses and infants are not likely to be exposed to asbestos through the placenta or breast milk of the mother. Results of animal studies do not indicate that exposure to asbestos is likely to result in birth defects. 12

Chrysotile, or white asbestos. Smithsonian National Museum of Natural History
The Asbestos Hazard Emergency Response Act (AHERA) was signed into law in October 1986. AHERA requires "schools to inspect their buildings for asbestos and take appropriate abatement actions using qualified, accredited persons for inspection and abatement." AHERA also requires asbestos control professionals to take appropriate initial training with annual refresher training (U.S. Environmental Protection Agency, 2008)… In 1989 the U.S. EPA issued a ruling banning most asbestos containing products. This ban was overturned by the 5th Circuit Court of Appeals in 1991. However, under this ruling certain products remained banned, including flooring felt, rollboard, and corrugated, commercial, or specialty paper. The ruling also maintained the ban on asbestos in products that did not historically contain asbestos (U.S. Environmental Protection Agency, 2008). 13
A new asbestos ban was introduced to congress in 2007. Senate bill S. 742, the "Ban Asbestos in America Act of 2007," was introduced on March 1, 2007 and passed unanimously on October 4, 2007 (GovTrack.us, 2007). The bill "Prohibits the importation, manufacture, processing and distribution of products containing asbestos." In addition to the six currently regulated asbestos minerals, it also defines asbestos as "any material formerly classified as tremolite, including winchite asbestos and richterite asbestos" plus "any fibrous amphibole mineral" (U.S. Senate, 2007). The House of Representatives version of the bill, H.R. 3285, was introduced on August 1, 2007. To date, the only action the House has taken on this bill was a hearing by a subcommittee on Environment and Hazardous Materials on February 28, 2008 (Committee on Energy and Commerce, 2008). 14
Mercury exists in several forms, but is generally classified into three categories: metal mercury, inorganic mercury, and organic mercury. Because it is in its pure form, metallic mercury is also known as elemental mercury. It is silver-colored, shiny, and liquid at room temperature. At room temperature (and warmer) some amount of liquid mercury will vaporize, the vapors have no odor or color. Once in the air, mercury vapors can travel great distances. Elemental mercury can combine with other elements (chlorine, oxygen, and sulfur, for example), forming inorganic compounds called mercury salts. Most mercury salts are white powders or crystals at room temperature, the exception being mercuric sulfide, which is red and turns black when exposed to light. Mercuric sulfide is commonly known as cinnabar. Cinnabar ore is mined and refined to extract the metallic form of mercury. The refining process involves heating the ore above 1,000 degrees Fahrenheit, which vaporizes the mercury in the ore. The vapors are then captured and cooled, forming liquid metal mercury. Organic mercury compounds are formed when mercury combines with carbon. The most common organic mercury compound found in the environment is methylmercury (also called monomethylmercury). Dimethylmercury and phenylmercury are found in very small amounts, but, like methylmercury, they are also very harmful to animal life in the environment (including humans). Methylemercury forms when metallic mercury undergoes "complex processes that move and transform" it involving microorganisms in soil and water. 15
According to the 1999, Toxicological Report on Mercury, for the U.S. Department of Health and Human Services, metallic and inorganic mercury have been available in various products. Uses of metallic mercury include: thermometers, barometers, sphygmomanometers (used to measure blood pressure), wall thermostats, fluorescent light bulbs, electric light switches, middle and high school chemistry labs, and amalgam dental fillings. Various inorganic mercury compounds have been used in a wide range of products including: fungicides, skin-lightening cream, medicinal products (laxatives, teething powders), topical medicines, as preservatives in some prescription and OTC medicines, as well as color for paints and tattoo dyes. Many of the mercury compounds in these products have been replaced with much safer substances. Most use of organic mercury has been banned, but was used as antifungal in both interior and exterior paints as late as 1991. Mercury is still used in mining, smelting, fossil fuels, and comes from the incineration of solid waste.
Core samples and other data provide evidence that the current high concentrations of mercury are the result of industrialization. Background mercury, mercury that occurs naturally, is fairly well distributed and at low concentration levels in any given spot. Mercury concentrations in the environment that are the result of human activity continue to be added and recycled. Depending on the factors of location, concentration, climate, etc. mercury in any of the three categories can be transformed into another. As a result, it is extremely difficult to measure what is being reintroduced to the environment.
Routes of exposure to the three categories of mercury include air, land, and water. Once in the air, mercury particles may remain suspended for as long as two years and be carried over great distances. 16 These particles are primarily the result of industrial emissions. Metallic mercury in the air can be transformed into either inorganic or organic mercury, reaching soil or water in the environment. Inorganic compounds contained in fungicides can be introduced into soil, but reach various water sources as a result of runoff. Microorganisms convert inorganic mercury to methylmercury and release it to soil or water. In soil, methylmercury may attach to small particles and remain for a long time. In water, any form of mercury will most likely settle and remain.
For each category of mercury, there are differing, specific routes of exposure. The difference in exposure type affect the potential health hazard because different organs and systems in the body respond differently to each category. For example, when swallowed very little metallic mercury is taken in by a healthy body; however, inhaled mercury vapors result in nearly 80% entering the bloodstream from the lungs and rapidly spreading throughout the body (including the brain and kidneys), where it can remain for weeks or months. 17 Inorganic mercury enters the body more from swallowing than inhalation, but like metallic mercury also tends to accumulate in the brain and kidneys, leaving the body weeks or months later via urine or feces. Like metallic mercury, organic mercury compounds can evaporate slowly at room temperature, the vapors easily being breathed into the body.
A source of exposure to metallic mercury comes from the amalgam (a mixture of chemicals) in dental fillings. In a hardened filling, mercury is bound to the other metals, "but very small amounts are slowly released from the surface of the filling due to corrosion or chewing or grinding motions…(and) may enter the air as mercury vapor or be dissolved in the saliva." 18 Of course, the amount released, and therefore the health risk involved, varies. Metallic mercury can be found in many products used indoors, so the handling of any indoor spills is very important. Because it vaporizes easily, very small amounts of liquid mercury can easily increase the concentration of mercury in air, creating a potentially on-going health hazard.
Exposure to inorganic mercury compounds is a concern for people in occupations with a higher than normal risk of contact in their workplace. Those occupations include employees where electrical equipment or automotive parts are manufactured, chemical and metal processing plants, construction workers. There is the additional risk that the clothing of these workers can become contaminated with mercury, thereby potentially exposing their family members as well. The greatest concern for mercury exposure to the general population is through the presence of the organic compound methymercury in the food chain.
In an aquatic ecosystem, mercury is converted to methylmercury by bacteria and eaten by small organisms, which are in turn eaten by larger fish, and so on up the food chain. Because methylmercury is absorbed more quickly than it is released, it accumulates in each successively larger fish and the land animals (including people) that consume them. According to the EPA, there are many factors affecting methymercury concentrations in fish (including mercury concentration in the water, pH and temperature of the water, the amount of dissolved solids and organic matter present, presence of sulfur and other chemicals), making the prediction of bioaccumulation difficult to predict. 19 They also state in the same document, "The concentrations of methymercury in large fish can be over a million-fold larger than in the surrounding water."
There are differences among U.S. agencies and the World Health Organization on the reference dose (an estimate of the daily amount of a substance that can be safely consumed over an individual's lifetime), 0.1 micrograms/kilogram of body weight per day (U.S. EPA), 0.4 micrograms/kg of body weight per day (U.S. FDA), 0.5 micrograms/kg of body weight per day (ATSDR), and 1.6 micrograms/kg of body weight per day (WHO). The Environmental Protection Agency has applies the most conservative safety factor, accounting for it having the lowest reference dose.
At Hunters Point Naval Shipyard elemental mercury is a concern in two areas: parcel B and parcel F. As stated earlier, parcel F includes the aquatic portion of the shipyard, surrounding the shipyard land. For study, parcel F was subdivided into eleven sections. Following studies, subdivisions I, III, VIII, IX, and X were identified as needing some form of remedial action. Area weighted averages of copper, mercury and total PCBs were derived for each of the five areas. For mercury those results were: India Basin Area I: 0.37mg/kg, Point Avisadero Area III: 3.8 mg/kg; Eastern Wetland Area VIII: 0.45 mg/kg; Oil Reclamation Area IX: 0.49 mg/kg, and South Basin Area X: 0.78 mg/kg. 20
Methylmercury is classified as a neurotoxin (poisonous to the nervous system) and a reproductive toxin (damaging to the reproductive system and/or developing fetus), and as a possible carcinogen (IARC). Ingested methylmercury is highly absorbed through the gastrointestinal tract (~95%). From the bloodstream, it easily enters body tissue, particularly the brain. Methylmercury can pass from the blood of a pregnant woman to that of her fetus, into its brain and other body tissues. It can also be passed from mother to infant via breast milk.
Studies have been done through animal testing. The testing indicates that methylmercury damages the developing central nervous system and that effects worsen with age, whether or not the individual's exposure has stopped. According to the Toxicological Report on Mercury, "Animals exposed orally to long-term, high levels of methymercury or phenylmercury in laboratory studies experienced damage to the kidneys, stomach, and large intestine; changes in blood pressure and heart rate; adverse effects on the developing fetus, sperm, and male reproductive organs; and increases in number of spontaneous abortions and stillbirths." 21 In a 2000 report to Congress, The National Academy of Science concluded that "the population at highest risk is the children of women who consume large amounts of fish and seafood during pregnancy, and that the risk to that population is likely to be sufficient to result in an increase in the number or children who have to struggle to keep up in school and who might require remedial classes or special education."
In addition to pregnant women, nursing mothers, women of child-bearing age, and children below the age of six years who are generally at risk, there are others in the Hunters Point community who are at risk due to cultural and/or economic factors. The Asian population of Hunters Point is approximately 14%, they may be at greater risk than the average because of their generally higher consumption rate of fish and shellfish (60-138 grams/day). Along with Hunters Point's poor Asian immigrants, others at risk due to subsistence fishing include African Americans, Native Americans, Pacific Islanders, and Eskimos. 22 Always, the specific risks are related to actual intensity, frequency, and duration of exposure, along with the age, gender, and physical condition of the individual consumers.
Unlike other toxins that accumulate in the skin or fat tissue of fish, mercury concentrates in the muscle tissue, so it is not possible to cook or fillet it out; the focus of government agencies currently it to provide recommendations and warnings about the types and amounts of fish to eat and/or avoid eating. The U.S. EPA and FDA make recommendations to who might become pregnant, are pregnant, are nursing mothers, and for young children that include: not eating shark, swordfish, king mackerel, or tilefish; eating up to 12 ounces a week of a variety of fish and shellfish that tend to be lower in mercury (shrimp, canned light tuna, salmon, Pollock, and catfish). California's Office of Environmental Health Hazard Assessment issued an interim advisory in 2007. The state advisory adds to the National EPA recommendations that women of childbearing age, are pregnant, nursing mothers, and children should not eat more than one meal of fish per month, should not eat any striped bass over 27 inches, and should not eat any shark.
The city and county of San Francisco has several Mercury Prevention Programs administered through its environmental department, the purpose of which is to reduce risk to residents of exposure and assist businesses in safely disposing of mercury waste and choosing low mercury alternatives. The programs include: thermometer exchange and fluorescent lamp collection for residents, dental mercury reduction (to ensure that mercury from amalgam fillings gets properly recycled) and fluorescent lamp outreach for businesses, and in-house City programs that include use of low-mercury and long-life lamps and use of rechargeable batteries where possible and recycling all types of batteries. 23
As part of this unit, I hope to be able to take the students on a field trip to the Hunters Point Shipyard. Additionally, perhaps someone from the regional office of the Environmental Protection Agency will be willing to make a presentation to explain the government's process for cleaning up toxic sites in general and our shipyard in particular. The regional office is located in San Francisco. In the absence of those two options, I am certain to be able to find someone locally qualified to speak on the topic. In preparation for a classroom speaker on the subject, students will develop questions in writing and practice asking them orally. First, they will participate in small groups in a vocabulary review of the key terms they will need to use in forming their questions. The vocabulary I've chosen to emphasize for all students is: concentration, dose, route of exposure, molecule, hazard, toxic, ingestion, absorption, element, population, inhalation, and response. As I have many students whose first language is not English, classroom activities focusing on key vocabulary are critical for students' conceptual development. The vocabulary review jigsaw activity I use comes from the QTEL (Quality Teaching for English Learners) program of WestEd corporation. Students work in groups of four to review twelve vocabulary terms. Each student numbers a sheet of paper from 1 to 12 and is given a card with a different type of clue on it. The cards are labeled A, B, C, and D. Card A lists the first letter(s) for each term, card B tells the number of syllables, Card C gives the last letter of the terms, and card D gives a definition/description of each term. The student with card A chooses any of the vocabulary terms, they need not be done in order, tells which number s/he has chosen, and reads the clue. The group works together to name each term as they go through the clues. After three terms, the cards are rotated to a new group member, giving each of them the opportunity to provide a different type of clue. When the group is in agreement on all of the terms, they raise their hands to get a copy of the answer card to check their work. Following the vocabulary review, students next task is a think-write-pair-share. Each student will consider what they have already learned and develop two questions. They may develop clarifying questions as well as "next step" questions. They may also choose to ask about the training, working conditions, etc. of the speaker. With their partner, students take turns reading their questions aloud, correct grammar, etc. As the partners are satisfied with their questions, they take them to be approved by the teacher. When everyone is finished, the questions are cut into strips and placed in a container, each student then pulls out a question strip that s/he will be able to ask the guest speaker. In this way students may or may not ask their own question of the speaker. For many, this increases the comfort level of speaking in front of the other students. For any students who really want to ask their own question, they may choose to do so.
I am curious as to what the difference is, if any, in the pH level of the water at different points along the bay, specifically whether it is significantly more acidic near the Shipyard. Our school district has digital probe equipment that can be checked out by teachers to use for lab work with their students. I have selected ten different locations along the east side of San Francisco to collect bay water from once a month, the students will test the pH level for each site and compare the results. If there are significant differences, students will need to make inferences as to the possible causes. In preparation for this, students will conduct a somewhat open-ended lab where they will have access to several substances, red cabbage indicator and the pH probeware. This will allow them to look at different methods of testing, finding ways to quantify qualitative results, recording and analyzing qualitative versus quantitative data, to compare results of different types of testing, and to practice with the pH sensors before we begin testing water samples. A couple days before this lab, students will be shown some of the substances they may test, and be encouraged to bring in a small sample of something from home that they would like to test. This is not a requirement since many students' home life may not allow for it for economic reasons; on the other hand, it creates buy-in for others so it usually doesn't create a problem and sometimes students will bring an entire package of a substance (shampoo, for example) and not want to take it home, so it becomes a donation to the department. The day before the actual lab, part of the period is spent reviewing the general procedure for testing with the cabbage juice and the pH sensor, discussing ways to clearly record results, and clarifying the general safety and housekeeping procedures for this particular lab. Fortunately, my school district has enough sensors that group size can be small, only 2 - 3 students per group. Doing this ahead of time allows for maximum testing during the actual lab, and also makes it easier for the teacher to give more encouragement and less direct instruction during the lab, providing the scaffolding that provides enough comfort with the procedures for the students that they can be more strongly urged to identify what they want to know and figure out ways to get the information. Fortunately for me (and them), my ELL-identified students are programmed together in their core curriculum classes and their language arts and math teacher are working with me on this unit to provide support in writing and organizing date (specifically, creating tables and graphs) in those classes. I will need to supply this support myself with the regular education classes, while the honors class students will be expected to organize their data as homework. At every class level, the class following the lab will include the presentation and discussion of what the students learned, an evaluation of how the lab went, and what questions they now have. This is also a good time to discuss precision and accuracy again.
One of the concerns for the inadvertent transport of toxins at Hunters Point Shipyard is via groundwater runoff. Three-fourths of the solid surface of the shipyard is due to landfill which came from within the shipyard itself, that soil already containing known as well as unknown toxins. The fill-in took place over years, so the quality of the landfill at any given point was dependent on what had been buried in or leeched into the soil before it was moved. Some of what was dumped or buried on the site is known, paint and various solvents, for example. Filling in of the Bay has been an ongoing environmental safety debate in the region for many years, and will most likely continue to be, as my students become adults, so this is a topic they should become increasingly aware of and develop opinions about as they move from adolescence to adulthood. Because of this, the unit will include an investigation of porosity and permeability of different types of soil. 24 Again, this will be a two-day lesson. Begin with a demonstration of porosity, using marbles, colored water, a beaker, and a graduated cylinder. Fill the beaker with marbles, and then ask the students if the beaker is full, most will say yes. Then add colored water from a graduated cylinder until the water level is equal to the marble level. Note the amount of water that filled the pore spaces between the marbles. Do a "think-pair-share" activity asking students to predict how sand, clay, and potting soil might compare in a similar test. Randomly, call on a few of the pairs to share their prediction with the class, allowing for one or two to volunteer their prediction afterward. Review the definitions of porosity and permeability, assign lab partners, show the students the supplies and equipment they will have to work with (those students with camera phones may take pictures of the set-up), and have the partners use the remainder of the period to work on their procedure. Students are asked to consider the following questions while working on their procedure: Will tapping the container cause the material to settle? Does this increase or decrease the porosity? Does this increase or decrease the permeability? Because of our tectonic activity, this is an important question for Bay Area residents. How does the shape of the grains affect porosity and permeability? How does the sediment grain size affect porosity and permeability? 25 Obviously, it is too dangerous for students to handle soil samples from Hunters Point Shipyard. However, students will spend a class period examining the "Toxic Substances Hydrology (Toxics) Program" (http://toxics.usgs.gov/index.html) of the USGS (U.S. Geological Survey) following the soil samples lab.
There is a great deal of concern in the neighborhood adjacent to Hunters Point Shipyard about how the clean-up and development projects of the shipyard, and the released area that is currently being developed by Lennar Corporation, are affecting air quality, whether or not residents are being adequately protected from exposure to such air-borne toxins as asbestos. To familiarize students with air quality testing and the question of particulate matter, students will complete a lab sampling and analyzing air pollution. This lab comes from two sources: "How to Sample Black Carbon Air Pollution Using a Vacuum Cleaner," (http://www.lbl.gov/Education/ELSI/Frames/pollution-measure-BC-f.html) from Lawrence Berkeley National Laboratory's ELSI Program (Ethical, Legal, and Social Issues in Science), and "Inventory Control: Sampling and Analyzing Air Pollution," from the Journal of Chemical Education (Volume 71 Number 4 April 1994, pp. 318 - 322). The Journal of Chemical Education article has the same vacuum cleaner set-up as the Berkeley Lab site, but also gives a setup using an aquarium pump. The aquarium pump setup has two versions to create an airtight seal for the soda bottle housing, a "wet" version and a "dry" version. Current and past issues/articles are available online, http://jchemed.chem.wisc.edu/index.html, however access to past articles is limited to current subscribers and the journal is generally not geared to middle school curriculum.
This unit lends itself to many investigative questions—science questions, history and social science questions, etc. I fully expect that through the process of teaching it this year, it will evolve in ways currently unpredictable and possibly unimaginable to me.
Notes
1. Toxins in the sandblast waste could vary depending on the time period, during the 1940's the shipyard was responsible for decontamination of the ships involved in the nuclear testing conducted at the Bikini Atoll, so blasting from this time period would include radionuclides in addition to the paint contents, including heavy metals.
2. Final Radiological Addendum to the Revised Feasibility Study for Parcel D April 11, 2008 DCN: ECSD-2201-0006-0078
3. California EPA ID# CA1170090087 http://yosemite.epa.gov/r9/sfund/r9sfdocw.nsf/vwsoalphabetic/Hunters+Point+Naval+Shipyard!OpenDocument&ExpandSection=-7
4. Hunters Point Shipyard: Historic Radiological Assessment. Naval Facilities Engineering Command, Southwest Division. PDF 2004 pg. 11.
5. Toxicological Profile for Asbestos. U.S. Department of Health and Human Services. Public Health Service Agency for Toxic Substances and Disease Registry. September 2001
6. Toxicological Profile for Asbestos. 1.1 What is Asbestos U.S. Department of Health and Human Services. Public Health Service Agency for Toxic Substances and Disease Registry. September 2001. Pp.2-3
7. ibid. pg
8. Mesothelioma: Questions and Answers. National Cancer Institute Fact Sheet. http://www.cancer.gov/cancertopics/factsheet/Sites-Types/mesothelioma
9. Toxicological Profile for Asbestos. 1.1 What is Asbestos U.S. Department of Health and Human Services. Public Health Service Agency for Toxic Substances and Disease Registry. September 2001.
10. Mesothelioma: Questions and Answers. National Cancer Institute Fact Sheet. http://www.cancer.gov/cancertopics/factsheet/Sites-Types/mesothelioma
11. ibid. pg.
12. Toxicological Profile for Asbestos. 1.1 What is Asbestos U.S. Department of Health and Human Services. Public Health Service Agency for Toxic Substances and Disease Registry. September 2001.
13. Asbestos in the United States: Occurrences, Use and Control by Andreas Saldivar & Vicki Soto. April 2008 ProQuest Discovery Guides http://www.csa.com/discoveryguides/discoveryguides-main.php
14. ibid.
15. USGS. Mercury in the Environment: fact sheet 146-00. October 2000 http://www.usgs.gov/themes/factsheet/146-00/.
16. Toxicological Report on Mercury
17. ibid. pg. 11
18. ibid. pg. 6
19. http://www.epa.gov/mercury/exposure.htm. Methylmercury exposure. Last updated on Friday, June 6th, 2008.
20. Feasibility Study Report for Parcel F Hunters Point Shipyard. San Francisco, CA. April 30, 2008. Barajas & Associates, Inc. 839 W. Harbor Drive, Suite 1 San Diego, California 92101
21. Toxicological Report on Mercury. Pg 15
22. Public Health Assessment. Naval Station Treasure Island: Hunters Point Annex. ATSDR. September 30, 2004.
23. http://www.sfenvironment.org/index/html.
24. Adapted from: Toxic Leak!: An Event-Based Science Module. Addison-Wesley Publishing Company. Menlo Park, CA. 1996.
25. Toxic Leak!: An Event-Based Science Module. Addison-Wesley Publishing Company. Menlo Park, CA. 1996.

Comments: