Background
Chemistry as Central Science
Study of chemistry involves chemical changes in our body and around us. One major focus is on the structure and properties of substances. Chemistry is very important as it serves as interface to all of the other sciences and many areas of human endeavor. Hence, chemistry is often called as "Central Science".
The language of chemistry includes symbols for elements, formulas for compounds, and equations for chemical reactions. The short hand representation of an element is called symbol. These symbols are recognized by International union of Pure and Applied Chemistry. Example: carbon is represented by C, sodium is by Na and xenon by Xe. The periodic table is used to catalog the symbols of elements.
The chemical formula indicates the relative numbers of atoms of the elements of a substance4. For example: the chemical formula for baking soda (sodium bicarbonate) is NaHCO 3. The formula tells that 1 atom of sodium, 1 atom of hydrogen and 3 atoms of oxygen are present in one formula unit of baking soda. Propane, C 3H 8, is a common fuel used for cooking and home heating. In the chemical formula the numbers 3 and 8 are called the subscripts and they represent the number atoms/moles of carbon and hydrogen in one molecule/mole of propane.
Chemical formulas are of two types. The empirical formula is the simplest formula that gives the correct relative numbers of atoms of each element in a compound, where as the molecular formula specifies the number of atoms of each element in a molecule of that substance. For example, the molecular formula of glucose is C 6H 1 2O 6, the ratio of atoms of carbon, hydrogen and oxygen is 6:12:6. The empirical formula of glucose is CH 2O, the atoms of carbon, hydrogen and oxygen are in the ratio of 1:2:1.
In a chemical reaction the reactants are converted to products. During a chemical reaction, atoms are neither created nor destroyed; they are merely reorganized. A chemical equation represents the chemical reaction showing reactants on the left side of an arrow and products on the right.
N 2 + 3H 2 -> 2NH 3
The reactants in the above equation are nitrogen (N 2) and hydrogen (H 2) where as the product is ammonia (NH 3).
Food Chemistry
The chemistry of food includes water and three essential macronutrients - carbohydrates, proteins, and fats. There are also many important components of food that add to our health but are only present in small quantities; these components are called micronutrients and include vitamins, minerals like calcium, potassium, iron and many others.
Nutritionists recommend that a healthy adult woman consume ~2000 cal of energy per day. Of course, this number can change, depending on the level of activity of the woman. These total calories should come from a mixture of macronutrients: for example, carbohydrate intake might be ~60% of calories, fat intake ~30%, and protein intake ~10% per day. Experts disagree about the exact percentages that constitute a healthy diet, but these percentages are a good starting point.
A calorie is the unit of energy, which is defined as the amount of heat required to raise the temperature of one liter of water from 14.5 o C to 15.5 o C. This is equal to the amount of energy a 150 pound person burns each minute while sleeping5. The body converts food into energy. The human body can make four calories of energy for every gram of carbohydrate, nine calories per gram of fat and four gram per gram of protein. Choosing perfect proportions and correct combinations can be easier with knowledge about nutrition and simple arithmetic.
Carbohydrates
Carbohydrates, commonly known as sugars, are made of carbon, hydrogen and oxygen with a general chemical formula C n(H 2O) n. This formula represents hydrates of carbon, hence the name was given as carbohydrates. Though it is now known that there are no full water molecules attached to carbohydrates, still the name has stayed. Carbohydrates contain multiple hydroxyl (-OH) and carbonyl (>C=O) functional groups. Carbohydrate molecules range in size from monomers (monosaccharide) to polymers (polysaccharides). Polymer (poly= many, mer = unit) is large a molecule with many repeating units called monomers.
Monosaccharides are the simplest carbohydrates and are referred as simple sugars. The most common monosaccharides, glucose, fructose and galactose, have either five or six carbon atoms. The presence of many polar groups makes these monosaccharides water soluble. Glucose is a six carbon sugar that has an aldehyde structure. Glucose is often called blood sugar as it is present in the blood at high concentration. It serves as major source of immediate energy. Fructose is a six carbon sugar with ketone structure. Fructose is known as fruit sugar as it is present in many fruits. Glucose and fructose are structural isomers, structurally different with same molecular formula6. Galactose is a stereo isomer of glucose. They differ in special arrangement of hydrogen atom and hydroxyl group around one of the six carbon atoms.
A disaccharide is formed when two monosaccharides are bonded together via a condensation reaction with the release of a water molecule. Sucrose and lactose are common disaccharides. Sucrose is also known as table sugar as it is mainly used as a sweetener. Sucrose is formed from glucose and fructose. Lactose is often called as milk sugar as it is important carbohydrate in milk. Lactose is formed from glucose and galactose is bonded together. After ingestion, disaccharides are too large to be absorbed into the blood stream directly, so the digestive enzymes sucrase and lactase break sucrose and lactose respectively into their monosaccharide units.
Polysaccharides contain 12 or more monosaccharide units bonded together. These are often termed complex sugars. Starch, glycogen and cellulose are important polysaccharides. Plants make starch and cellulose: starch is water soluble where as cellulose is insoluble. The animal counterpart of starch is a different polysacchride called glycogen. It is made by animals to store energy, mostly in muscles and liver. Glucose is the monomer for each of these three polymers. Although they have same monomer unit, they have different properties. This is because of the way the glucose monomers are bonded differ in the three polysacchrides. Cellulose has a linear structure which resembles a chain like fence. Starch molecules are either branched or unbranched, and glycogen is highly branched. Due to the difference in their bond shape, humans can digest starch and glycogen but not cellulose.
Digestive enzymes can't fit cellulose into their active sites due to the specific lock and key fit needed for enzyme action. As a result, the cellulose in the fruits, vegetables, and grains that we eat passes through the digestive system without being changed or absorbed. Molecules that behave like this are called dietary fiber.
The main function of carbohydrates is as a source of energy, both immediate and stored. When carbohydrates are oxidized they release carbon dioxide, water and energy. Foods that are rich sources of carbohydrates include bread, rice, pasta, potatoes, milk, pie, soft drinks, vegetables, fruits etc7. Because of the difference in the composition of the carbohydrates in each of these foods, their short and long-term effects on energy in the body differ.
Eating whole grain products helps the body's sugar control system8. Insulin, which is produced by pancreatic beta cells, is secreted into the blood circulation in response to the rise in blood glucose after meals. Insulin regulates blood glucose levels by suppressing glucose production from the liver and stimulating glucose uptake by cells throughout the body. When glucose (or other simple sugars) are eaten directly, blood sugar and therefore insulin rises dramatically. The fiber in whole grains leads to a slower rise in blood glucose and eases the workload for the insulin making cells in the pancreas9. Diabetes occurs when the pancreas is unable to secrete insulin—or cells in the body stop responding to insulin.
Proteins
The word protein came from the Greek root word protos, which means first10. Proteins are organic polymers made of amino acids linked together in a specific way. Each amino acid has a carboxyl and an amino group. The amino and carboxyl groups provide convenient bonding sites for linking amino acids together. The amide bond that joins two amino acids is known as a peptide bond. Proteins are not just large molecules but also randomly arranged chains of amino acids. There are 20 amino acids, which make up the tens of thousands of different proteins in our body. Our body makes some of these amino acids and rest are obtained from food. These amino acids are referred as essential amino acids as they are essential in the diet.
Proteins are the building blocks of the body. Proteins play many roles in our body. Proteins are involved in forming structures, digesting foods, catalyzing reactions, transporting substances, regulating cellular processes, recycling wastes, and even serving as an energy source when other sources are scarce. For example, insulin is a protein hormone, a small protein with 51 amino acids.
The recommended daily allowance of protein is 50 grams a day for a 140 pound person and almost 65 grams for a 180 pound person11. Common sources of protein are meat, milk, nuts, fish, and some fruits and vegetables. Protein is found in the body in high concentrations in muscle, hair, skin, bone and all other tissues. The effects of dietary proteins on health probably are approximately the same for animal protein and plant protein. Animal proteins tend to be complete, as they are sources of all essential amino acids. However one must be careful about eating too much of it, as animal protein tends to come with saturated fat. Though vegetable proteins are incomplete, that is, they do not have all essential amino acids, but still they are good source of proteins. Research says that eating a lot of protein does not harm the heart12. Choosing the right protein sources that are low in saturated fat will help you keep in good health.
Fats
Fats are large, non polar, biological molecules. Fats are insoluble in water as they are non polar. Fats have two major functions in living organisms. They store energy efficiently, and they make up most of the structure of cel1 membranes. Fats are convenient source of energy storage. Our dietary fat contains phospholipids, and cholesterol in addition to triglycerides. A triglyceride is formed by condensation of one molecule of glycerol with three molecules of fatty acid. Animal and vegetable fats are complex mixtures of triglycerides. The cell membrane is made up of phospholipids that regulate transportation of substances across the cell membrane. Our body requires cholesterol to make estrogen, testosterone and other vital compounds.
There are four types of fatty acids: monounsaturated, polyunsaturated, saturated and trans. All fatty acids are long chain hydrocarbons. Unsaturated fatty acids contain double bonds between some of the carbon atoms. Depending on the number of double bonds, the fatty acid can be monounsaturated (one double bond) or polyunsaturated (more than one double bond). Due to the cis orientation of double bonds naturally occurring in unsaturated fatty acids, they have a kink or bend that prevents them from packing together efficiently. This results in less intermolecular attractions, and lower melting points. Unsaturated fatty acids are in liquid phase at room temperature. These fatty acids are termed as good fats because eating these fats instead saturated fats and carbohydrates lowers levels of low-density lipoprotein (bad) cholesterol with out lowering the levels HDL (good or protective) cholesterol. Olive oil, vegetable oil, and fish oils are rich in unsaturated fats.
Saturated fatty acids do not contain double bonds hence they are saturated with hydrogen. Saturated fatty acids can pack together due to their straight chain structure. Saturated fatty acids have higher melting points, hence they are in solid form at room temperature. Whole milk, red meat, and coconut oil are good sources of saturated fats. These fats are termed as bad fats as they strongly increase the LDL (bad) cholesterol13. Hydrogenation, addition of hydrogen, to unsaturated fatty acids yields saturated fatty acids. For example, oleic acid can be hydrogenated to form stearic acid.
Trans fats are mostly man made fats. Polyunsaturated fatty acids upon partial hydrogenation yield trans acids. During this process, hydrogen will be added on to double bonded carbons, but not all, to create single bonds. At the same time, some of the remaining double bonds change their orientation, from cis to trans, resulting in new physical and chemical properties to fats. Like saturated fats, trans fats increase the LDL cholesterol. They also elevate the triglycerides and lipoproteins. A higher level of these in the blood stream increases the chances of heart disease. Trans fats not only increases the LDL levels but also decreases the HDL (protective form) levels. This does not happen with saturated fats. This indicates that trans fats are more dangerous than saturated fats. Vegetable shortenings, most margarine, deep fried fast food, most commercially baked foods, and partially hydrogenated vegetable oil14 are sources of trans fat.
Including the good fats in the diet and keeping away the bad fats keeps a person healthy. In the recommended 30% of dietary calories, less than 1/3 should be saturated fats and rest of them should be unsaturated fats15. Most importantly, keep trans fats out of your meal.
Balancing Equations
The Law of Conservation of Mass states that 'the mass of the universe is constant'. This means that mass is neither created nor destroyed. According to the Law of Conservation of Mass, one has to balance every chemical equation so that the mass of substances remain the same before and after the chemical change. Another way of stating this, which is more convenient for chemists, is: all atoms present in the reactants must be accounted for among the products. A balanced equation gives the relative numbers of reactants and product molecules. In a balanced chemical equation the subscripts tell the number of atoms of each element in a molecule, where as the coefficients tell the number of molecules/moles of reactants and products.
As mentioned earlier, in our body several chemical reactions take place during digestion, respiration and other processes. Some examples of biochemical reactions:
Example1: During cellular respiration, our cells make energy from the breaking down of glucose by oxygen into carbon dioxide and water. The process is an exothermic process, the energy releasing process.
C6H12O6 + 6O2 -> 6 CO2 + 6H2O + Energy
Example 2: The hydrolysis of sucrose into glucose and fructose
C12H22O11 + H2O -> C6H12O6 + C6H12O6
Example 3: Peptides are synthesized by coupling of carboxylic group of one amino acid with amino group of another amino acid to form peptide bond.
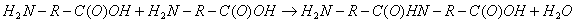
Example 4: Fermentation of sugars into alcohol:
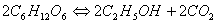
A fraction of chemical reactions that occur in our body during metabolism, cellular respiration, and protein synthesis is mentioned above to show the importance of chemistry in understanding the metabolism, and our food.
Mole Concept
We are all familiar with measuring the quantity of substances by their mass: I have one pound of oranges or 10 grams of gold. But there is another way of measuring amounts that is convenient for chemists, or anyone interested in substances that can react. A mole is a unit of measure equal to the number of carbon atoms in exactly 12 grams of pure Carbon -12. A mole of any other substance is this same number of units of that substance.
One mole of any substance contains Avogadro's number of units of that substance. Avogadro's number has been determined experimentally to be 6.022 X 10^23, which is a large number. The molar mass of a compound is the mass in grams of one mole of the compound and is computed by summing the average masses of its constituent atoms.
As I mentioned in the rationale, a stoichiometry problem requires the understanding the mole concept, molar mass, balancing equations, and conversions. As a mole is such a big number, I use Mole Facts16 to fascinate my students. Some of the mole facts are listed here:
- 6.02 X 10^23 Donut Holes: Would cover the earth and be 5 miles (8 km) deep.
- 6.02 X 10^23 Watermelon Seeds: Would be found inside a melon slightly larger than the moon.
- 6.02 X 10^23 Grains of Sand: Would be more than all of the sand on Miami Beach.
- 1 Liter bottle of Water contains 55.5 moles H 2O
- 5 Pound Bag of Sugar contains 6.6 moles of C 1 2H 2 2O 1 1 (Sucrose)
Stoichiometry
Stoichiometry (from the Greek stoicheion, element, and metria, science of measurement)17 deals with the calculation of the quantities of material consumed and produced in chemical reactions. It is like chemical arithmetic. Stoichiometry is used in industry quite often to determine the amount of materials required to produce the desired amount of products in a given useful equation. Stoichiometry calculations help scientists and engineers working in industry to estimate the amount of products they will obtain from a given procedure: it can also help decide whether the product is profitable to produce or not.
Companies make many chemical substances, through chemical reactions, that are helpful in our lives. For example, addition of stannous fluoride, SnF 2 , to tooth paste to prevent the tooth decay in tooth paste industry; aspartame, a sugar substitute, in soft drinks in soft drink industry; preparation of citric acid from the fermentation of sugars (sucrose) in air in food industry; synthesis of aspirin in pharmaceutical industry; use of titanium metal and its alloys in aerospace industry; extraction of titanium from its ore rutile, TiO 2, in metallurgy; production of the bleaching agent, calcium hypochlorite, from sodium hydroxide, calcium hydroxide, and chlorine in detergent industry; manufacture of polyethylene (which is found in some milk cartons) in polymer industry; removal of dangerous mercury compounds from industrial waste in environmental chemistry. The list could go on. Each one of these products requires stoichiometry.
There would be no products from these industries without chemical stoichiometry. Amounts of reactants consumed and products formed can be calculated from the balanced equation for a reaction by using the mole ratios relating the reactants and products. In this unit we will concentrate on understanding and making use of these mass relations.
Limiting Reactant/ Limiting Reagent
The limiting reactant is the one which is consumed first and hence determines the amount of products that can be formed. The other reactants in the chemical reaction are called excess reactants. The excess amount of these reactants will be left over, without reacting, when the reaction is complete. For example, if you are hungry, the number of grilled cheese sandwiches you can make depends on how many slices of bread and slices of cheese you have. You can make five sandwiches from sixteen bread slices and five cheese slices. You could not make eight sandwiches, even though you had sixteen slices of bread, because you had only five slices of cheese. In this situation, the limiting reactant is cheese and excess reactant is bread. Your products are five sandwiches and six slices of bread (which is the unreacted excess substance).
Percentage Yield
The theoretical yield of a product is the maximum amount that can be produced from a given amount of limiting reactant. The actual yield, the amount of product actually obtained in a given experiment, is always less than the theoretical yield. The ratio of actual yield to theoretical yield, multiplied by 100%, gives the percentage yield in a given reaction. In the above mentioned example about making sandwiches, if for some reason (say, accidentally, a cheese slice fell on the floor) you could make four sandwiches instead five, then the percentage yield will be (4/5) 100% = 80%.
Percent yield = (Actual yield/ theoretical yield) 100%

Comments: