Background
Photosynthesis
Humans and all living things depend on photosynthesis for nourishment. Photosynthesis completes many of our needs. It provides fiber and building materials. It fills the need for energy by storing it in petroleum, natural gas, coal, and even firewood. This is also the process that produces the oxygen that makes up a portion of the earth's atmosphere. Plants, some forms of bacteria and algae produce food by this process. These autotrophs are capable of synthesizing food directly from inorganic compounds, like carbon dioxide and water to produce sugar and oxygen gas. Later, when plants need food, they draw upon the energy stored in these sugars.
In photosynthesis, light energy from the sun is captured using the pigment chlorophyll. These pigments are contained in organelles called chloroplasts. Although all green parts of plants have chloroplasts, most of the energy is produced in the leaves. The cells in the interior part of a leaf, called the mesophyll, contain about half a million chloroplasts for every square millimeter of leaf. 7
Photosynthesis, as shown in Figure 1, occurs in two phases, the light dependent and the light-independent reactions. Light dependent reactions convert light energy to chemical energy in ATP (adenosine triphosphate). The energy in ATP is used to produce simple sugars in light-independent reactions. The general equation for photosynthesis is:
6CO 2 + 6H 2O → C 6H 1 2O 6 + 6O 2
The first phase of photosynthesis needs sunlight. When sunlight strikes the chlorophyll in the leaves, energy from light is transferred to the electrons in the chlorophyll molecule. The energized electrons are passed from chlorophyll to a series of electron acceptors in the thylakoid membrane, which is called the electron transport chain. Each electron acceptor in the chain passes energized electrons to the next one. At each step, the electrons lose energy. Some of the energy is then used to pump hydrogen ions into the center of the thylakoid disc. After traveling along the electron transport chain from photosystem II to photosystem I, the electrons get re-energized and are transferred along a second electron transport chain. At the end of the second chain, the electrons are still energized. Finally, the electrons are transferred to the stroma of the chloroplasts, where an electron carrier, NADP + (nicotinamide adenine dinucleotide phosphate) is used. NADP + combines with two energized electrons and a hydrogen ion (H +) to form NADPH. NADPH stores the chemical energy that will then be used in the light-independent reactions.
Used electrons in the chlorophyll molecule are replaced by breaking the water molecule. For every water molecule that is broken, one half molecule of oxygen, two electrons, and two hydrogen ions are formed. The oxygen is released in the atmosphere. The electrons are returned to the chlorophyll while the hydrogen ions are pumped into the thylakoid where they build up in high concentration. With the concentration difference across the thylakoid membrane, hydrogen ions can be transferred across the thylakoid membrane and provide energy for the production of ATP.
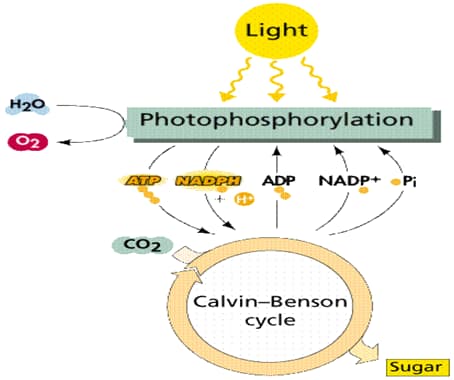
Figure 1: The Process of Photosynthesis. 8
The second phase of photosynthesis does not require light. It takes place in the stroma of the chloroplast. The carbon in CO 2 is added to one molecule of RuBP (Ribulose biphosphate) via the carboxylase enzyme, a very abundant protein in the chloroplast, and form sugar through a series of reactions in the stroma. The NADPH and ATP produced during the light reaction are used in the cycle.
The process of photosynthesis is a perfect example of atom economy where both reactants, (water and carbon dioxide) are all turned into products (sugar and oxygen gas). This process also gives scientists the needed background to look for more sustainable ways that energy could be generated, so that society would have alternative sources of power for man's various needs.
Photosynthesis and Bioaccumulation in Food Chain and Food Webs
Energy moves from plants and some forms of algae and bacteria to animals. This energy moving up the food chain comes from the sun. Animals cannot get energy directly from the sun so they need help from smaller living things that can make their own food. In the Arctic Ocean, for example, little greenish organisms called phytoplankton are the energy sources. They can, like ordinary plants, synthesize food from water and carbon dioxide. From these phytoplankton, the sun's energy moves up the chain. Phytoplankton are usually eaten by zooplankton, the zooplankton are eaten by arctic cod, and the arctic cod are eaten by seals, whales, or sea birds. Then, bigger animals like the polar bears will feed on some of them, like the seals. In the arctic region, the northern people eat wildlife, so humans also occupy the top of the chain. As energy moves up the food chain, there is less and less energy available for use.
An opposite scenario can be observed as hazardous chemicals move up the food chain. When a toxin is picked up by smaller organisms and passed it on to bigger predators, bioaccumulation causes an increasing concentration. Because many toxins are hydrophobic, they do not mix or dissolve in water. They cannot be excreted or flushed out. So, these substances build up in fatty tissues and magnify. An example of this phenomenon is found among the plankton that absorbed about 5 parts per million of the insecticide DDD in Clear Lake, California. Plant-eating fishes had built up accumulations ranging from 40 to 300 parts per million; carnivorous species like the brown bullhead had an astounding concentration of 2,500 parts per million. 9 Bioaccumulation is seen greater among the predators at the top of the food chain.
Bioaccumulation of Toxic Substances
Life begins with photosynthesis. Nutrition and energy flow begin in this process. This allows plants and other photosynthetic organisms to get nutrients necessary for survival. Humans rely mostly on plants to have a constant supply of natural vitamins and minerals. But eating greens does not always mean eating healthy. Having fruits and vitamins in our diet does not always mean freedom from toxic chemicals. Trees, foliage, and plants flourish because farmers often use substances like pesticides. And these chemicals persist and concentrate in living cells. This is bioaccumulation. This is how toxins enter the food chain. Pesticides are especially interesting chemicals because, first, they include a very diverse set of synthetic and natural toxins that include insecticides, fungicides, rodenticides, herbicides, and algaecides; second, because they must be normally be released into the environments to be effective, which makes contamination of water, air, soil, and wildlife difficult to control. 10
In 1798, Thomas Robert Malthus, an English clergyman and economist, published an essay about populations inevitably tending to grow faster than their food supply. This indeed happened. Since Malthus' time, the world population has increased from less than 1 billion to over 6 billion. 11 The need for food escalated, and agricultural scientists looked for ways to increase food production. A German chemist, Justus Von Liebig, (1803-1873), pioneered in identifying chemical substances that plants require for healthy growth. 1 2 As a result of his work, farmers began increased amounts of natural fertilizers to supply plants with the needed nutrients. The need for increasing amount of fertilizer led directly to the introduction of synthetic inorganic fertilizers.
Chemical use in farming grew across the continents. In the Philippines, the increased production of rice that made the country one of the major exporters of rice in Asia was due to the use of chemical farming, an initiative started by the International Rice Research Institute in the 1960's. 13 The Institute was home to the Green Revolution that led the region to rapidly increase rice yields and overall production.
Agriculture business grew, and the law of supply and demand resulted in the transportation of plants and produce brought from one place to another. The importation and exportation of plants and plant products caused the spread of pests. One example was the Colorado potato beetle that was brought by wagons to Illinois in the 1860's. 1 4 The spread of pests affected not only the local breed of crops in an area but those that were imported as well. There was a disruption of ecosystems. As a result, crop prices fell, the economy was hit, and the world faced massive health-related problems. This phenomenon paved the way for chemical use to kill pests and insects. It started in the 1870's when Paris Green was put to tests against the potato beetle. 15 Then, other substances were introduced like the mixtures of sulfur and lime sprayed in orchards. The failure of other methods to meet public demands for ways to stops insects without long, expensive research, changing farming practices, or long-term planning paved the way for the triumph of chemicals. In less than two decades of their use, the synthetic pesticides have been so thoroughly distributed throughout the animate and inanimate world that they occur virtually everywhere. 16 And now, it can be noted that there has been a tremendous increase in the production of synthetic pesticides from the time they were introduced in the market until today.
Pesticides
A pesticide is any substance or mixture intended to preventing, destroying, and mitigating pests. A pesticide can be natural or synthetic. An example of a natural pesticide is a pheromone, while DDT is the most well known synthetic pesticide. Pesticides do not only include insecticides. There are other kinds serving the same purpose but targeting different kinds of living organisms. In the United States, the Environmental Protection Agency (EPA) is responsible for regulating the production and use of pesticides. The introduction of synthetic pesticides has helped made U.S. agriculture a profitable enterprise. But often, certain species developed resistance to the chemical. The total use of pesticides in 1997 was approximately 4.6 billion pounds of active ingredients, and in the same year, more than 500 species of insects and more than 150 types of fungi were found resistant to some pesticides. 17 As a result, pesticides were combined, applications were increased, and more toxic and ecologically hazardous pesticides were used. Nearly 325 active ingredients of pesticides are permitted for use in 675 different forms of food, and residues of these compounds are allowed by law to persist at the dinner table. 18
Both pesticides and insecticides were seen effective in increasing yield and making produce fresh looking and enticing in grocery stalls and markets. One very well known insecticide is DDT. In 1945, it was first utilized against potato beetles and cloth's moths and later became synonymous to insect control. 19 But, studies conducted after that indicated that the chemical could accumulate in living tissues because it was fat-soluble. It was also observed to be very persistent and mobile because residues were found in cow's milk even if the substance was sprayed in empty barns. Residues also linger, lodge, and magnify in the bodies of organisms that are higher in the food chain. Consider looking at the building up of these poisons. They pollute the air, the lands, and the waters. Their residues linger and become more potent and powerful as they magnify and extend beyond limits of contamination. Think about the risks we take as these substances accumulate in different plant parts and move through various trophic levels. In humans, they are not only poisons; they destroy enzymes and prevent normal functioning of body systems. According to various studies, individuals with no known exposure store an average of 5.3 parts per million to 7.4 parts per million. Agricultural workers had an average of 17.1 parts per million and workers in insecticide factories had a very high 648 parts per million. 20
Heavy Metals
Another reason why insecticides and pesticides were considered threats to humans and to the environment is they often contain heavy metals like mercury, copper, nickel, lead, chromium, and cadmium. 21 These heavy metals have atomic masses greater than the masses of those of the useful metals like potassium, calcium, and magnesium. Their ions are also noted for toxicities. They are the chemical elements with a specific gravity that is at least 5x the specific gravity of water. Other scientists defined these as those with elemental densities above 7 g/cm 3. 22 They are found naturally in the earth's crust and can enter the human body in very small amounts through the food, water and air. They are important as trace minerals but, at greater amounts, they can lead to poisoning. There are also some nonmetals like arsenic and selenium that have the ability to magnify in living systems. In West Bengal, India, where 800,000 people drank well water containing over 50 mg/L arsenic, 200,000 people developed skin lesions from drinking the water. In Bangladesh, 70 million people drink well water containing arsenic and they exhibited similar symptoms. 23
The periodic table in Figure 2 below, shows metals classified as: Class a: hard metals; Class b: soft metals; and Borderline: intermediate metals. The terms "hard" and "soft" were used to denote the kinds of acids the metals formed. Hard metals form hard acids. Hard acids are compounds characterized by ionic bonds. These compounds are found mobile in living systems, and can be easily displaced or flushed out. Soft metals, on the other hand, form soft acids that have covalent bonds. Soft acids are compounds that are observed immobile and accumulate in living systems with resultant toxicity. The metals included in the Borderline category exhibit Class a and Class b properties. The heavy metals discussed in my unit are mostly found under Class B and Borderline. Some fall under both categories; like, copper may be either Class b or Borderline depending upon whether it is Cu(I) or Cu(II), respectively; lead may be either Class b or Borderline depending upon whether it is Pb(II) or Pb(IV), respectively; and iron may be either Class a or Borderline depending upon it is Fe(III) or Fe(II), respectively.
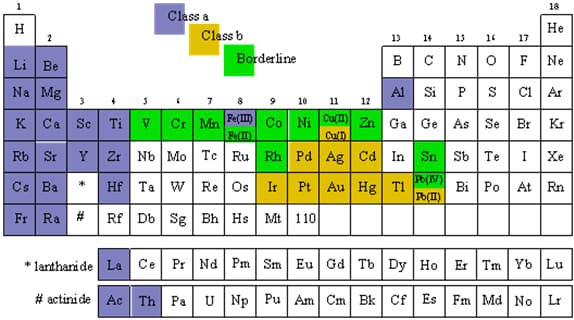
Figure 2: Periodic Table Classified as to Classes (Heavy Metals in Class b, and Borderline). 2 4
Chromium: In one of my discussions with a friend, we agreed that the moment we are born, we are already contaminated with many substances. We live in contaminated environments, using contaminated products. Heavy metal contamination is observed all throughout the globe. Look at the Superfund Sites in Alameda California. Alameda Naval Station is a facility built to provide materials support to the navy. Wastes discharged from the manufacturing facilities are discharged to Seaplane Lagoon and to two on-base landfills. These wastes included industrial solvents, pesticides, cyanides, and the metal chromium. 25
Chromium (Cr) has atomic mass of 51.996 amu. It can be hexavalent, a form that is toxic, mutagenic, and carcinogenic. Saying so, wide spread of chromium in the environment poses a serious threat to humans and animals. Its chromate ion (CrO 4 - 2) has high solubility in soil and ground water and tends to be mobile. It is actively transported in cells where it was found to be capable of causing lesions in DNA. 26 Hexavalent chromium (Cr + 6) is also found to cause irritation to the lining of the nose, nose ulcers, and breathing problems. Animals exposed to the ion showed sperm damage. 27
Lead: Lead has the symbol Pb coming from the Latin name Plumbum. Its atomic mass is 207.2 amu. It is the most recycled metal and it is used in the manufacture of many things like alloys, pigments, and ammunition. Lead is also important in making pottery, automobile electrical storage batteries, solder, cooking vessels, pesticides and paints. Lead exposure and contamination can come from food and water. Lead water pipes are still in existence in some countries even if they can cause lead poisoning. Lead can also come from the air when it is added to gasoline to produce better burning automobile fuel. The lead enters the atmosphere through automobile exhaust as lead oxide. Lead can adversely affect children and women of childbearing age. In the past, its durability made it an excellent paint additive; thus, at one time, baby cribs were painted with lead-based paints. The sweetness was a temptation for young children and this resulted in infant deaths and other illnesses. Children are more susceptible to lead because they are much smaller and will receive a much higher dose given the same exposure. The Center for Disease and Control assigned the unit mg/ dL to be the common biomarker of lead exposure in the blood lead level. For adult male workers, the level of concern is at 40 mg/ dL. For children, it is noted to be 10 mg/ dL. 2 8 Toxicity symptoms include lowered IQ, memory, and learning difficulties, behavioral problems and developmental and nervous system disorders. 29
Mercury: Mercury is the only metallic liquid element and has an atomic mass of 200.59 amu. Its symbol is Hg from hydrargyrum, meaning quick silver. The major source of mercury is the earth's crust, during volcanic emissions. It is an ingredient for many consumer products, an excellent electrical conductor, is found in medical and weather thermometers, thermostats, mercury-vapor street lamps, fluorescent bulbs, and paints. It is also famous for dental amalgams. There are two forms of mercury, the inorganic and the organic (methyl) mercury. Mercury compounds are toxic, but they are useful in eliminating bacteria, fungi, and agricultural pests when used in antiseptics, fungicides, and pesticides. Inorganic mercury can be absorbed as liquid and its vapor is hazardous because it gets converted to methyl mercury. Mercury is a global environmental pollutant. Its forms can bioaccumulate especially in fish and enter the food chain. Its minimal risk level is at 0.2 mg/m 3 and permissible exposure limit is 0.05 mg/m 3. 30 Symptoms of mercury contamination are severe effects to the nervous system with developmental issues that include cerebral palsy-like signs with involvement of the visual, sensory, and auditory systems, tingling around lips, mouth, fingers, and toes, vision and hearing loss. 31
There had been tremendous cases of mercury poisoning since the Minimata incident. Chemical and manufacturing facilities in Japan in the 1950's contaminated the fishes in Minimata Bay, Japan. Thousands of cases of mercury poisoning had been documented since then around the world. In Iraq alone, there were about 40,000 cases when some 73,000 tons of wheat and 22,000 tons of barley intended for planting and treated with organic mercurials were made into flour. This irresponsible incident caused about 6500 deaths. 32 Mercury contamination is, sad to say, still ongoing in various continents.
Cadmium: Cadmium (Cd), with an atomic mass of 112.41 amu, has properties very similar to zinc. Since zinc is an essential micronutrient for plants, animals, and humans, cadmium can sometimes enter the food chain and get absorbed by an organism. In humans, it is believed to cause renal failure if there is long-term exposure. High exposure can also lead to lung disease, and bone defects. In animals, it is linked to increased blood pressure and myocardium effects. The average daily intake for human is 0.15 mg from air and 1.0 mg from water. Smoking a packet of 20 cigarettes can lead to the inhalation of 2 - 4 mg of cadmium, but levels may vary widely. 33
Cadmium is produced as a by-product of zinc production. This metal is used in nickel/cadmium batteries, coatings, pigments, and stabilizers for PVC, in alloys, and electronic compounds. It is present as an impurity in several products, like phosphate fertilizers, detergents, and refined petroleum products. Cadmium contamination showed up among workers of Huizhou PP Battery Factory in Hong Kong where there were about 3000 workers affected. This incidence affirmed what researchers had observed on cadmium exposure that once in the body, it will take 7 to 30 years to get the toxins flushed out. 34
Antimony: We are always exposed to antimony (Sb from Stibium) because this element with an atomic mass of 121.76 amu is found in most paper products and rubber. This metal can also be found in batteries, pigments, plastics, and ceramics. Antimony is a very valuable component in alloy making because of its strength. When exposed to high concentration of antimony, organisms are observed to exhibit nausea, vomiting, and diarrhea. It is a suspected carcinogen. Short-term exposure by inhalation in humans results in effects on the skin and the eyes. Long-term exposure can lead to inflammation of the lungs, chronic bronchitis, and chronic emphysema. 35 The Environmental Protection Agency (EPA) has established the Reference Concentration (RfC) of antimony to 0.0002 mg/m 3. 36 This RfC measure was based on the effects of contamination on rats. Exposure greater than the RfC value has the potential for adverse effects to increase.
Copper: Copper (Cu from Cuprum), a metallic element with an atomic mass of 63.55 amu is essential to plant, animal, and human lives. In high dosage, it may cause anemia, and liver and kidney disorders in humans. It is mostly found in air, food, fungicides, and drinking water because of copper pipes. This also comes from additives that prevent algal growth. EPA requires that the copper level in drinking water be less than 1.3 mg/L. 37 The recommended daily allowance (RDA) in our diets should not be more than 900 mg/day and the Occupational Safety and Health Administration (OSHA) requires the amount of copper not exceed 0.1 mg/m 3 in air and 1.0 mg/m 3 for copper dusts. 38
Copper contamination of various bodies of water in the United States began a long time ago. Mining was such an invaluable way for the country's economy to grow and studies done showed water reservoirs flooded with mine workings. Lakes and rivers in Northern parts of California were analyzed in 1987 and copper and zinc concentration particularly coming from the Penn Mine to the Mokelumne River reached to about 1,000 ppm. 39
Nickel: Nickel (Ni), with atomic mass 58.69 amu, is released as dust in refineries, and is very useful in metallurgical processes. It is an electrical component in Ni-Cd batteries. It is naturally occurring in rivers, lakes, and in plants and animals. Nickel is needed in the human body in very small amount but when taken orally in excessive dosages, it becomes acutely toxic. Reproductive and developmental effects in humans are at 0.13 to 0.20 mg/m 3. 40 Short-term exposure is not that dangerous but long-term exposure can cause decreased body weight, heart and liver damage, and skin irritation.
Solution Concentration
Solutions have two parts, the solute and the solvent. We are most familiar with solutions in which a solid is dissolved in a liquid but there are as many types of solutions as there are different combinations of solid, liquid, and gas. We have solid, liquid, and gaseous solutions. A clear example of a solid solution is an alloy, whereas we know that the atmosphere is a good example of a gaseous solution. The mixture of vinegar and water is a liquid solution.
The concentration of a solution is defined as the amount of solute present in a given amount of solvent. This concept is a constant part of our daily experiences. Making coffee or tea, preparing beverages, adding antifreeze to automobiles, or mixing pesticides in the soil we use for gardening apply the principle of solution concentration. This is seen even in simple cooking. It is affected by changing the amount of either the solute or the solvent. There are different ways to describe the amount of solute in a given solvent. We use the terms dilute or concentrated but these only give us a rough qualitative idea of concentration.
Quantitative description is found more effective in describing solution concentration. Because solutions can have varying composition, the relative amounts of substances must be specified. There are some ways we can express solution concentration quantitatively; and these are: mass percent, mole fraction, molarity, and molality.

Comments: