Background Information
I have been teaching much of the background information that follows, but during my research of the topic, I learned the answers to many of the questions I had about how neurons work, how imaging techniques of the brain are done, how disease affects the brain, and how plastic the brain is. In addition, as I was looking for research examples for my students to use, I learned a great deal more about what we still do not understand about the brain. So read on for the things your biology text left out about the nervous system!
The nervous system is divided into the central nervous system (CNS), containing the brain and spinal cord, and the peripheral nervous system (PNS), which connects the CNS to the rest of the body. The basic unit of the nervous system is the neuron. Its function is to transmit signals to other neurons, muscle cells, or glands. The integration of signals, carried out by the central nervous system, is used to regulate body processes, and perceive and respond to the world around us.
Neurons
Although there are many types of neurons, they (almost) all have some similar characteristics. Each neuron has a cell body or soma containing the nucleus of the cell and other organelles necessary for carrying out the cell's function. Extending from the cell body are two types of processes (cellular extensions). A neuron may have one or many dendrites, which are branching processes that receive incoming signals. One axon, often with branching ends, leaves the cell body. It is this special process that sends an electrical signal—the action potential—which is transmitted down its length to the next cell. At the terminating end of the axon is a gap, the synapse, between it and the target cell.
Before beginning a discussion of how an action potential is propagated, it is a good idea to review membrane structure, the properties of the lipid bilayer, membrane proteins, and passive and active transport across the membrane with students. Students should recall that ions cannot cross the lipid bilayer, but must cross through transmembrane proteins. Some of these proteins are simple gated channels, which change shape to allow the ions through, and some are pumps which require energy in the form of ATP to pump ions across the membrane against their concentration gradient. Some of these channels are permeable to cations, some to anions, and some are specific to only one type of ion. 1 Some of these channels are open for longer periods of time or open more frequently, such as K + and Cl - channels, and must be deactivated to close. Others are more likely to be found closed, and must be activated to open. Some channels are stretch-activated, and open when the cell membrane is mechanically disturbed. Other channels are voltage-gated and open or close in response to changes in membrane potential. Still others are ligand-activated, meaning a specific chemical, called a ligand, binds to the membrane protein, either extracellularly or intracellularly, causing it to open. An example of this kind of activation is when calcium channels in the postsynaptic membranes of muscle cells are opened by the binding of the neurotransmitter acetylcholine. 2 The important point is that when these channels open and allow ions through, the concentration of ions inside and outside the cell changes, changing the membrane potential.
In addition to ion channels, other proteins in the membrane of neurons—along dendrites, the cell body, or even the axon—are important as receptors for neurotransmitters released by other neurons. The binding of these neurotransmitters, can lead to a change in the membrane potential. 3 Some neurons may have many such connections with other neurons, especially in the brain, where the development of connections between neurons is crucial to how the brain functions.
Membrane potential refers to a difference in charge across the cell membrane. The cytoplasm of the neuron is filled with proteins, anions such Cl -, and cations such as Na +, K +, and Ca 2 +, but is generally negative compared to the ionic concentration of the extracellular fluid. The membrane is permeable to potassium ions, with some open ion channels, and potassium reaches a state of equilibrium between the concentration gradient driving it to diffuse out and the potential gradient attracting the ions into the cell. (The K+ ions will diffuse out, down their concentration gradient, but are also being pulled in because they are attracted to the negatively charged interior of the cell.) At rest, the concentration of potassium ions is greater inside the neuron [120mM] than in the extracellular fluid outside the neuron [4mM]. The concentration of sodium ions outside the cell [145mM] is greater than inside the cell [15mM]. This sets up an electrochemical gradient for sodium ions to potentially enter the cell if more sodium channels open. 4 The resting potential of most neurons is about -70 millivolts, meaning the inside of the cell has a potential 70 millivolts less than the extracellular fluid. That is the starting point, or resting potential, for some stimulus to begin the events that lead to an action potential.
Propagation of the action potential is clearly described in most AP Biology textbooks. What is often not clear is how the signal is propagated from the dendrites, across the cell body, and to the axon. Bring in the Localized Graded Potential (LGP)! It is localized because it is a change in the membrane potential in one small area; graded, because the amount of change in the potential varies according to the strength of the stimulus. (This will not be the case with an action potential! Action potentials can be propagated over long distances with no variation in the amount of change in the potential, or its duration. 5) A localized graded potential is initiated by some stimulus. As one example, neurotransmitters released at a synapse between an interneuron and the dendrite of a motor neuron will produce an LGP when ion channels open in the membrane of a dendrite, raising the membrane potential, and depolarizing a small area of the cell. 6 However, the change only lasts as long as the neurotransmitter is bound to the receptor. 7 Since neurons have very high resistance to the conduction of electricity, if no further input is provided, the current fades out quickly. This change in membrane potential would never make it down the axon, but since the distance that it must travel down the dendrite or through the cell body is very short, it may make it to the beginning of the axon, the hillock. 8 The cumulative effect of multiple LGPs contributed by multiple dendrites, may result in enough voltage-gated sodium channels opening that the threshold membrane potential is reached, triggering an action potential at the hillock of the axon. At the hillock, there is a higher concentration of sodium channels and a lower threshold for the initiation of an action potential. (So that's why the hillock is important!) Action potentials may also start in dendrites and lead to the initiation of an action potential in the axon, or in cell bodies, where the threshold level is higher. 9
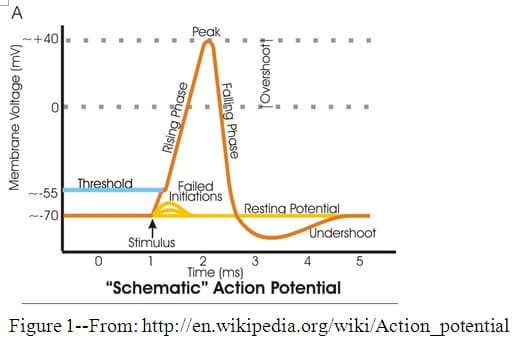
When the threshold level has been reached at the proximal end of the axon, usually about -55 millivolts, voltage-gated sodium channels change shape due to the change in charge and open, allowing sodium ions outside the cell to diffuse rapidly down their electrochemical gradient into a small section of the axon. (See Figure 1.) The inward flow of sodium causes the membrane potential to rise, becoming positive for a short time. This rising phase of the action potential is called depolarization. At this point the membrane potential has been reversed: the inside of the axon is now positively charged and the outside is now negatively charged. When the membrane potential reaches its peak, about +40mV, the voltage-gated sodium channels begin to close, but voltage-gated potassium channels open, allowing K + ions to rush out down their concentration gradient and down the new potential gradient toward the negatively charged exterior of the axon. As more potassium ions diffuse out of the cell, the membrane potential falls, and the potential reverses again. This phase is called repolarization. During a brief period of time, called the refractory period, sodium channels are inactivated by the change in potential, and are unable to be opened by another threshold potential. So many potassium ions diffuse out through, not only the normally open K + channels, but also the voltage-gated K + channels, that the membrane potential drops below its original resting potential, resulting in a brief period of hyperpolarization. During this time the voltage-gated K+ channels close. The original resting potential is restored as potassium ions reestablish their equilibrium. Resting membrane potential is restored, but the position of the ions has been reversed, with more Na+ ions on the inside and more K+ ions on the outside of the axon than there were at the resting state. Although it does not take a lot of these ions to cross the membrane for these voltage changes to occur, the ions must be returned to re-establish equilibrium. They are pumped back across by another membrane protein, the sodium-potassium pump. This protein pump works continuously throughout the action potential, and the periods between the action potentials, to actively pump three sodium ions out for every two potassium ions it brings in, restoring the ionic concentrations to their original state. 10 At this point, this location in the axon is reset and ready to receive another stimulus.
When the first action potential occurs at the axon hillock, the localized change in membrane potential caused by depolarization, raises the membrane potential in adjacent sections of the axon, downstream from the original axonal section that was stimulated, initiating a new action potential there that is as strong as the previous one. This section's action potential initiates an action potential in the next section and so on. In this sequential fashion, action potentials are propagated all the way down the axon. Action potentials travel faster in axons with wider diameters, such as the giant axons in squid. The reason for this is that the propagation of the action potentials down the axon is dependent on how far down the axon the next action potential can be initiated by the depolarization phase. The positive charge of the depolarization can travel locally through the axon or across the membrane through open membrane channels. A larger diameter axon has lower surface area to volume, and therefore more of the positive charge of the depolarization flows through the axon, resulting in depolarization of the membrane farther ahead of the action potential. 11
All axons cannot be big or we would need a much bigger skull to hold them. So axons have other mechanisms for speeding signal propagations. Some axons are insulated with a myelin sheath composed of Schwann cells, in peripheral neurons, and oligodendrocytes, in the central nervous system, wrapped around the axon at intervals. These glial cells produce an insulating fatty layer of myelin that surrounds axons and restricts the movement of ions into or out of the axon. Interspersed along the myelin sheath are sections of the axon that are not covered by the sheath, called the nodes of Ranvier. This intermittent insulating layer speeds up the movement of the action potential down the axon, because as the impulse passes through the part of the axon covered by the myelin, the potential is maintained because no ions can escape, and so it travels by diffusion the short distance covered by the myelin, to the nodes, where the action potential is propagated once again. 12,1 3 The speed of a signal down an axon varies depending on the diameter of the axon and whether or not the axon is myelinated, but the size of the action potential does not vary. The intensity of the stimulus that initiates the action potential is measured by the number of action potentials that occur in a neuron over time and is also indicated by the number of neurons firing. 14
When the action potential reaches the terminal end of the axon, the impulse is converted from an electrical signal to a chemical signal. At the axon terminal, the change in membrane potential triggers voltage-gated calcium channels to open, and calcium ions flood into the terminal. Here in the axon terminal, vesicles filled with neurotransmitters are waiting near the presynaptic membrane. When calcium ions enter the cytoplasm, proteins in the vesicle membranes change shape and fuse with the presynaptic membrane, forming a pore, through which the neurotransmitter is released into the synapse. The pore widens and the entire vesicle membrane is incorporated into the presynaptic membrane. Endocytosis of the membrane is used to reform vesicles which are refilled with neurotransmitters. When calcium channels are close to the site of vesicle-binding to the presynaptic membrane, neurotransmitter release is very fast. When they are farther away, release is slower and may even delay until a threshold level of calcium ions is reached. 15
After being released from vesicles, neurotransmitters diffuse the short distance across the synapse to the post-synaptic neuron. There are two types of neurotransmitter receptors that may be found on postsynaptic cells: transmitter-gated ion channels and G-protein-coupled receptors. When a neurotransmitter binds to a transmitter-gated ion channel, the channel changes shape slightly and opens to allow ions to diffuse into the postsynaptic neuron. If it allows sodium ions in, the resulting depolarization creates an excitatory post-synaptic potential (EPSP). The neurotransmitters acetylcholine and glutamate activate EPSP ion channels. If the channel that opens lets in Cl-, the resulting hyperpolarization creates an inhibitory post-synaptic potential (IPSP). The transmitter-gated channels that bind the neurotransmitters glycine and GABA cause an IPSP. 16 Both EPSPs and IPSPs are forms of localized graded potentials. The summative effect of the binding of different neurotransmitters from many different pre-synaptic neurons will result in the activation or inhibition of an action potential in the post-synaptic neuron.
G-protein-coupled receptors bind neurotransmitters and activate small molecules called G-proteins (guanosine triphosphate binding proteins) that move along on the inside of the post-synaptic membrane and activate either G-protein gated ion channels, or enzymes that produce second messengers which then trigger other enzymatic activities controlling ion channels or cell metabolism. 17 The neurotransmitter acetylcholine causes an EPSP in skeletal muscle cells, but is inhibitory in heart muscle and slows heart rate. The reason is that ACh binds to G-protein receptors in the membranes of heart muscle cells which activate G-proteins. These small proteins then bind to and open potassium channels, resulting in hyperpolarization of the muscle cells. 1 8 Activated G-proteins can also begin a cascade of enzymatic reactions inside the post-synaptic cell that result in the opening of many ion channels instead of just one, extending the signal to wider and more distant areas of the membrane. 19
To stop the signal to the post-synaptic cell, neurotransmitters must be removed from the synapse. Some simply diffuse away. Some are reabsorbed by transporter proteins in the membrane of the presynaptic neuron and either destroyed, or repackaged in vesicles to be used over again. Glial cells surrounding the neurons may also reabsorb neurotransmitters. The neurotransmitter acetylcholine is destroyed in the neuromuscular synapse by an enzyme, acetylcholinesterase, produced by postsynaptic muscle cells. Interaction with synaptic transmission is the focus of many drugs—which act to either enhance or block the transmission of the neurotransmitter across the synapse. 20
Evolution of the Brain
The only animals that lack any kind of nervous system are the sponges. Some, like the cnidarians, only have a network of neurons. Most invertebrates have a simple nerve cord with a cluster of neurons in the anterior region that serves as a coordinating region for the animals' responses to their environments. How brains evolved in vertebrate animals is still not fully understood. Brains, being soft tissue, do not fossilize. Therefore, fossil evidence of brain characteristics is mostly found by looking at casts of braincases. We can however, look at living vertebrates today and get an idea of the progression of brain development, and the different routes that it took in various lines.
All vertebrates share three basic divisions in the brain—the hindbrain, the midbrain, and the forebrain. Fossils of fish from 500 million years ago show these three areas of the brain. 21 The hindbrain includes the medulla oblongata, pons, and cerebellum, located at the top of the spinal cord. The medulla oblongata is at the top of the spinal cord; it regulates respiration, breathing and blood pressure. The pons relays information to the cortex and is involved in alertness. The cerebellum coordinates movement, posture and balance. It is larger in terrestrial animals than in fish. The hindbrain is dominant in fish, with the midbrain, devoted to the optic lobes, and the forebrain mostly composed of the olfactory bulbs. 22
In amphibians and reptiles, more of the sensory processing is done in the forebrain, which is divided into the diencephalon and the telencephalon. The diencephalon is composed of the thalamus, which integrates incoming sensory information and relays it to the cerebrum, and the hypothamus, which controls basic drives, emotions, and secretions from the pituitary gland. The telencephalon of reptiles and amphibians became the cerebrum in mammals. It is used for learning and memory. Birds have a larger cerebrum than reptiles, and in mammals, the cerebrum is the largest part of the brain. 23
The cerebrum is divided into two hemispheres, following the bilateral symmetry of all vertebrates. And yet there is evolutionary evidence for lateralization—the specialization of functions in the two hemispheres of the brain. The fact that the left side of the brain controls the right side of the body and vice versa, might show a kind of symmetry. The fact that there is a preponderance of right-handedness in humans would indicate lateralization of that control. Studies have shown that humans are not alone in being predominately right-handed. Other primates show a preference for using the right hand when performing many tasks. 24 Beyond handedness, there is evidence that the left and right sides of the brain have evolved to take on a wide range of different tasks. As vertebrate brains evolved, each hemisphere became more specialized for certain kinds of tasks. The left hemisphere took over tasks that involved ordinary patterns of behavior that were self-motivated, such as feeding and language. The right hemisphere was then left to deal with unexpected stimuli, and to take control when the environment threatened in some way, and a rapid response was needed. Many animals react more to predators that they see in their left field of vision—using the right side of the brain. 25 It makes sense that natural selection would have favored the compartmentalization of tasks in the brain rather than duplicating roles on both sides of the brain.
Humans have the largest brains in proportion to body mass of all vertebrates. The size of our cerebral cortex sets us apart from all other animals, even our closest relatives, the chimpanzees. 26 Animals are often used in neurological studies even though their brains are not nearly as complex as ours. There are differences in the size of various parts of the brain, but many of the structures are similar enough to make using animals to understand the workings of the brain valid. When trying to develop drugs and other treatments for neurological disorders, it is more cost-effective, not to mention safer, to test those treatments on animals first. However, results of studies with animals do not always translate readily to humans. 27
Imaging Systems
One difficulty with understanding the brain is that it is protected inside the skull, out of view, with hardly any discernibly moving parts. It is also such a complex network of so many kinds of neurons and supportive cells that pathways for processes are difficult to find and follow. Since Camillo Golgi developed his staining technique for neurons in 1873, scientists have been trying to find ways to get at the information stored in the brain. One way to understand how the brain works is to look at how it is different when it doesn't work. Brain lesions are damaged areas of the brain as a result of injury or disease. Lesion studies have helped us understand how some parts of the brain function, when abnormalities or injuries have affected individuals in particular ways. Paul Broca discovered a language center in the left hemisphere of the brain by studying a man whose speech was affected when he incurred damage to that area of the brain. 28 The prefrontal cortex was shown to control decision making and control of emotions when an iron rod passed through the head of Phineas Gage in a construction accident, leaving him alive, but forever changing his behavior. 29
Modern technology provides new ways to study brain function. An electroencephalogram (EEG) can be used to detect electrical activity in the brain and where that activity is located. An EEG is done by placing a network of electrodes over the head, which measure the electrical currents created by activation or timing of neuron signals in the cerebral cortex. The technique is noninvasive, and has been used in sleep studies, but is limited in its usefulness because the signal must pass through many layers to reach the electrodes, and the information generated is not very detailed. 30
Positron Emission Tomography, or PET scans, trace radioactive molecules injected into patients as the isotopes decay. The radiation given off by the isotope in the tracer molecules is detected in a scanner and converted into an image. Depending on the isotopes used, blood flow, metabolic reactions, protein synthesis, or neurotransmitter synthesis can be traced to see what areas of the brain are active. This technique can be used to study receptor changes in diseased neurons, to determine what the response of the brain is to a drug, or to determine what the optimal dosage is for a particular drug. PET scans may be used to find actively growing tumors, or to diagnose disorders. 31
Magnetic resonance imaging, or MRI, is a completely noninvasive technique that uses a combination of radio waves that pass through the brain, and a large magnet to create a detailed image of the soft tissues in the brain. Functional MRIs (fMRI) are used to map blood flow or oxygen consumption within the brain. Because fMRI can measure functional responses, like blood flow, they can be used to map activities in the brain, similar to PET scans.
At the cellular level, various dyes and staining techniques have been used to help visualize neurons. Antibodies designed to bind to specific proteins inside neurons, or on dendrites, axons or at synapses have been used to target and visualize particular processes in neurons. A gene for a bioluminescent protein from a jellyfish can be linked to promoters for the gene for GABA, injected into mice brains in order to visualize where the neurotransmitter is going in the brain.
Genetically altered "knock-out" mice, are designed to be deficient in a particular gene which gives them the characteristics of a human disorder, such as schizophrenia. These animals can then be used to study how the gene affects the nervous system and behavior, and how potential drugs or other treatments might alter these effects, before testing the drug on human patients. All of these new techniques offer hope for the understanding, treatment, and cure of nervous system disorders.

Comments: