Background
Non-Renewable Energy: Fossil Fuel Reserves
Currently the United States is dependent on fossil fuels as its main source of energy. The United States currently imports almost half of the oil consumed by the United States and a small percentage of natural gas which makes us increasingly dependent on foreign sources of fossil fuels and increasingly involved in the economic and political structures of the source country. In terms of energy use, seventy percent of the nation's electricity is generated through the burning of coal and natural gas, sources which are non-renewable. Non-renewable means that once the supply is depleted then it cannot be replaced, the glass is empty. In 2009 British Petroleum estimated worldwide fossil fuel reserves will supply, at current consumption, 205.0 thousand million tonnes of oil to last 46 more years, 6621.2 trillion cubic feet of natural gas to last 59 years, and finally 826,001 million tonnes of coal to last 118 years. Keep in mind that these estimates are based on current consumption. Every year as our world population increases so does our need for energy. This increase in population and energy consumption differ in that the world consumption of energy is increasing at a much faster pace than the population increase. This difference between the two indicates that the estimated fossil fuel reserves will not last as long as predicted. It is easily seen from this information that we are at a cross roads, we are running out of energy in the conventional sense from the utilization of fossil fuels. We have a few choices and we must convey the importance of these choices to our students. Although this section is for providing background information, I want to take a moment to diverge and underscore the importance of energy conservation. As opposed to waiting for the preplanned time in our science unit when we discuss energy or when the implementation of this curricular unit is appropriate in your teaching plans, I would suggest the following. Instead of beginning your energy unit by knocking your students over with a giant mallet and proclaiming that from this day forward they will now be budding environmentalists, you may prefer a more subtle, long lasting approach, a student friendly approach which involves student choices.
One simple choice which can be easily accomplished by every member of our society, especially our students, is to decrease our consumption of fossil fuels. As a teacher you can model this without ever having to explain what you are doing. As an example, as you go about your daily routines of teaching take a moment to evaluate how you personally can reduce your energy needs in your classroom environment. There are so many simple tasks which you can perform such as: turning off lights in empty rooms, controlling the thermostat in the classroom, if you have windows, use them and reduce the amount of artificial lighting in the room, and better monitoring and controlling of the amount of waste and recycling in the classroom. As the year progresses you will notice that these simple tasks become lifelong behaviors and you will be more energy efficient. And when you eventually begin this curricular unit or any unit involving energy, you will have a perfect springboard into the unit by having the students critique your energy usage in the classroom. If you can teach these concepts to your students through modeling, there is a better chance that they too will model these positive behaviors and begin them at home. If these simple energy reduction behaviors become the norm with the students' families, then I believe we can easily reduce our energy needs in as little as a semester and thus conserve energy and more importantly, begin to get students to evaluate their personal energy needs.
As opposed to reducing our use of energy, a second choice in meeting our increasing demand for energy would be to identify more reserves of coal, oil, and natural gas. We have identified and located all of the easily found, large reserves of natural gas, oil, and coal around the world, but what about the smaller reserves? Exploration and eventual extraction of these smaller reserves will be extremely cost intensive for companies. Coal mining may continue as humans and machines begin to mine at greater depths which greatly increase the costs and potential for loss of human life. Exploration at greater ocean depths, off shore, means greater water pressure and ocean current flow which produce their own unique set of problems. Drilling at greater depths on land requires equipment which can endure greater pressure and temperatures or require the use of the controversial method of natural gas and oil extraction known as "fracking."
Fossil Fuel Hazards
Fracking involves drilling into natural gas or oil reserves and injecting a highly pressurized fluid mixed with sand into the reservoir rock. Drilling may be straight down into the ground, or at any angle below ground, and drilling can be done horizontally along rock layers. With the increasing pressure in the bore hole, hydraulic induced fractures occur which allow oil or natural gas reserves to migrate through these "cracks" and then be extracted. All in all, the search for and extraction of smaller fossil fuel reserves will be extremely expensive compared to current fossil fuel prices. But it is expected that the ever increasing demand for fossil fuel generated energy combined with the dwindling reserves will make the extraction of fossil fuel from these smaller reserves very profitable. Two other extremely important concerns for continued fossil fuel dependency involve the political and environmental realms. As a search intensifies to locate the smaller reserves of natural gas, oil, and coal, companies may have to negotiate lucrative "mineral right" contracts with the countries who sit atop these fossil fuel reserves. This may prove to have a positive impact on countries that could really use the money to improve the standard of living in the country or, it could have the opposite effect in countries ruled by leaders who could use the increase in wealth to further their regimes. Not only are the problems associated with fossil fuels worldwide and national, but they cause a multitude of problems in California as well.
California electricity is derived from a variety of sources as depicted in Figure 1 for the 2007 year. Depending on the source, whether the electricity was generated from the burning of fossil fuels or from a renewable source, or combination of both, the average price per kilowatt hour of electricity in California was almost 15 cents for the month of March, 2012. 3 Although this may seem like a bargain to some, especially if you purchase electricity at this low price, it truly is not a bargain because of what is not included in the cost. The average consumer has little idea of the extent of environmental damaged created from the burning of fossil fuels; especially oil and coal. One of the far reaching, damaging byproducts from burning of fossil fuels is referred to as, "greenhouse gasses."
According to the Environmental Protection Agency (EPA) in 2010 the United States produced greenhouse gasses (GHG) composed of the following gasses and average percentages: Carbon Dioxide (CO 2), 84%; Methane (CH4), 10%; Nitrous Oxide (N 2O), 4%; and Fluorinated gasses, 2%, Figure 2. These gasses are referred to as greenhouse gases because they create the effect in the earth's atmosphere similar to the environment found in a greenhouse. Sunlight enters the earth's atmosphere and a portion is reflected back from the surface of the earth back up to the atmosphere as heat (infrared radiation). This reflected heat strikes the greenhouse gasses and much of it is absorbed or trapped and not allowed to leave the atmosphere, hence the slow warming of the earth's atmosphere which is changing our climate worldwide. The gasses are also responsible for air and water pollution, increasing acidity of the atmosphere, and the ever increasing, devastating effects to our health from particulate matter (PM), especially those classified as fine-particulate matter, released into the atmosphere from the burning of fossil fuels.
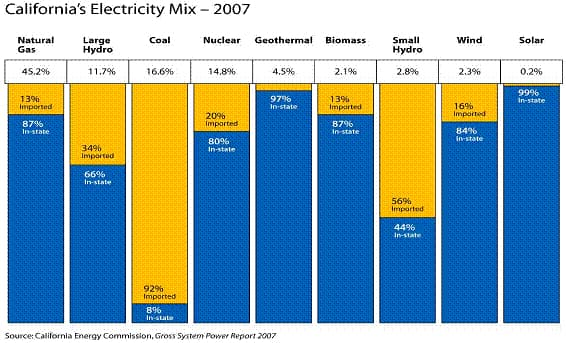
Figure 1 4
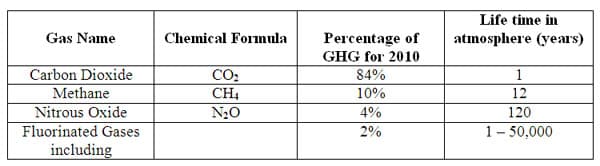
Figure 2 5
According to the EPA, coarse particulate matter is composed of particles with a diameter larger than 2.5 micrometers and less than 10 micrometers. The particles which have the greatest effect on human health are those particles which are labeled as "fine particulate matter" which have a diameter smaller than 2.5 micrometers. The reason that fine particulate particles are of great concern is the fact that they can be inhaled deep into the lungs where they may enter the bloodstream. Fine particulate matter is responsible for triggering heart attacks in people, especially fatal heart attacks in those who have a history of heart problems and cause an increase in difficulty breathing for those suffering from asthma. The very young and old are at the greatest risk from fine particulate matter and when cities have poor air quality days it is not uncommon for a dramatic increase in emergency room visits for respiratory related problems. But, coarse and fine particulate matter problems are just a few of the identified hazards associated with the burning of fossil fuels. Water and land pollution are of major concern especially as newer technologies such as fracking are implemented for the extraction of oil or natural gas from reserves which are not easily assessable by conventional recovery methods.
There are five major concerns associated with of the fracking process in terms of pollution and hazards. First is the concern over the huge quantities of water which are pumped out of ground water reservoirs to be used in the fracking process itself. The removal of these huge quantities of water may lower water tables and interfere with aquifers primarily used for drinking or agriculture. The second area of concern is possible pollution and contamination of surface areas from chemical spills or leaks during the mixing or injection of the chemical laden fluids to be pressure pumped underground. Thirdly, the injection and fracking process itself may pollute underground water aquifers. Fourth, contamination or spills from the recovery of the injected fluids used. The fifth concern deals with the treatment of the waste fluids from the entire process. Currently the EPA is in the process of collecting data related to these five major concerns and more information will be available through the EPA as data is collected and analyzed. Thus, even though the electricity in California was almost 15 cents per kilowatt hour in March, the actual cost is substantially higher from all of the environmental and health problems associated with burning fossil fuels. Please keep in mind that I believe we have not yet identified all possible health related issue arising from the burning of fossil fuels at this time. As we improve our technology and understanding of the human body, especially on the cellular level we may find a whole other host of possible problems. On a more positive note, the City of San Jose California is using newer technologies and is working diligently to make San Jose the most environmentally friendly city in the nation.
San Jose's Energy Consumption and Associated GHG Generation Electricity
In 2008 the City of San Jose consumed slightly over 5,400,000 Megawatts/hour (Mwh) of electricity with a resulting Carbon Dioxide pollution of over 1,570,000 Metric Tons of CO 2 (MTCO 2) as shown in Figure 3. One megawatt-hour of power is a large amount of power and as a means of putting this into perspective, consider the following scenario:
A large warehouse will be available for one of the largest book reading gatherings in the world. In anticipation of the arrival of the avid readers, 10,000 light bulbs rated at 100 watts each will be used to provide adequate lighting for the event. If all of the lights are left on for one hour, then 1,000,000 watt hours or 1 MWh or electricity will be consumed as calculated below.
10,000 light bulbs X 100 watts each X 1 hour of use = 1,000,000 watt hours
= 1,000 kilowatt hours = 1 megawatt hour.
In a similar fashion of relativity, consider the amount of volume contained in the 1,570,000 Metric Tons of CO 2 released into the atmosphere. It would occupy the equivalent volume of 833 Empire State Buildings.
Natural Gas
The consumption of natural gas was almost 6,400,000 thms with a resulting production of almost 2,800,000 Metric tons of CO 2 (MTCO 2) as shown in Figure 3. The Standard International unit of one therm (THM) may seem confusing but it is really not. Usually gases are measured or consumed in units of volume such as meters cubed or feet cubed but natural gas varies in its chemical composition. One cubic meter of gas from one location may contain substantially more impurities than an equivalent amount from another location. Because of this difference in "heat energy" per volume the "therm" was introduced in the United States in 1968 to better be able to accurately measure the heat energy of gas and charge the customer or consumer accordingly. The City of San Jose became very concerned with its energy use and resulting "carbon footprint" ( CO 2 ) and as a means to help reduce its dependency on fossil fuel generated electricity, improve the air quality and overall environment within the City, San Jose drafted, adopted, and passed one of the most comprehensive "Green" plans of any city in the country.
San Jose City's consumption of electricity and natural gas and associated production of CO2 for 2008
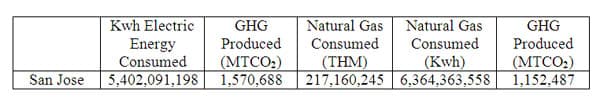
Figure 3 6
San Jose Power Plants
The City of San Jose receives most of its electricity from three major natural gas burning power plants. They are the Agnews Power Plant, Metcalf Energy Center, and Los Esteros Critical Energy Facility as shown in Figure 4. As of this writing, the Los Esteros Critical Energy Facility is undergoing remodeling in order to upgrade its' gas fired, simple-cycle system. The baseload values represent the constant or "baseload" supply of electricity which the power plant can generate continually under normal conditions. The "peaking" is the maximum amount of electricity the power plant can generate if needed during high demand situations. All three power plants burn natural gas, and like the burning of all fossil fuels for electricity generation, this process is very inefficient. Approximately one-third of the energy contained in the original unit of fossil fuel is converted to usable electricity provided to the end user. The huge loss of energy is found in the burning of the fossil fuel and the associated heat from that burning, the operation of the power plant itself, and the transmission of electricity through various sub-station and power stations prior to reaching the consumer.
After an extensive analysis of their energy consumption, greenhouse gas production, and a quickly increasing population, the City of San Jose decided to take action. They did not just go "green" but decided to be the leading city in the nation for green technology throughout the entire City.
San Jose, California Electricity Generating Power Plants
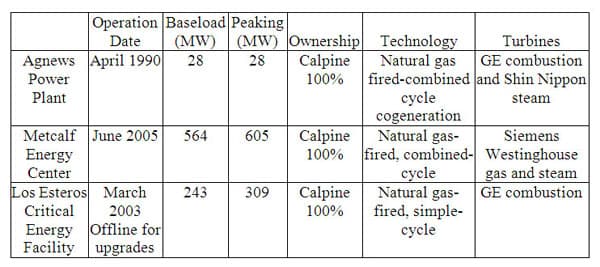
Figure 4 7
San Jose's Green Vision Plan Overview
In October of 2007, the City of San Jose released its' "San Jose's Green Vision" document. This document was the result of countless hours of research in order to determine the best way for the entire City to become green with a City sponsored plan covering every aspect of San Jose from City Government to new construction, and utilization of renewable sources of electricity. San Jose has received many awards and honors for the adoption of this plan and is recognized as has having the most ambitious plan of any city in the nation in an attempt to be the most innovative, creative, and successful city in the country in implementing "green" standards. San Jose's 10 goals are listed below in Figure 5. My curricular unit falls right into alignment with goal number 3 and stresses the importance of using electricity generated from renewable sources such as solar.
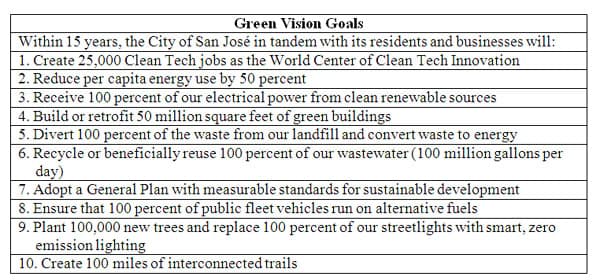
Figure 5 8
Renewable Energy
Photovoltaic Cell Design, Function, and Efficiency
The French Physicists, A. E. Bacquerel is credited as being the person responsible for building the first photovoltaic cell in 1839 at the age of 19. In 1905 Albert Einstein explained the "physics" behind the photovoltaic effect and received a Nobel Prize for his efforts. Over the years, many others continued working on the advancement of photovoltaic cells (PV) in an attempt to bring the cost of renewable electricity produced from a (PV) equivalent to the costs from obtaining electricity from fossil fuel. Consider that in 1955 Bell Laboratories perfected a photovoltaic cell which was 6% efficient. Bell Laboratories' cell produced one watt of electricity for $250 and electricity generated from the burning of coal cost a little over $3 for a watt at that time. Instead of just letting the entire photovoltaic cell sit as an interesting phenomenon, Bell Laboratories pushed forward to improve the efficiency and lower the costs of electricity produced by photovoltaic cells due to the promising use of the cells on satellites. Modern photovoltaic cells are much more efficient and are getting better, literally every day, as more and more financial resources are directed toward improving photovoltaic cell efficiency and lowering production costs. The average conversion of solar energy into usable electricity is approximately 18%, but new photovoltaic cell prototypes are averaging almost 40% efficiency which will greatly increase the amount of electricity produced by the cells. Most "basic" photovoltaic cells are made from silicon.
Silicon was one the earliest elements used for making photovoltaic cells and is still the most commonly used material today. Pure silicon is element number fourteen on the Periodic Table, consists of 14 protons and electrons, and is the second most abundant element on the earth. It has three energy levels with four valance electrons in the last energy level. In pure silicon, the outer four electrons of silicon share a bond with the outer four electrons of another silicon atom. In order to make pure silicon a "semi-conductor," small amounts of other elements are exchanged for silicon atoms in the silicon crystal structure in a process called, "doping." In a very simplified overview of how a photovoltaic cell is constructed, the cell begins as two extremely thin semiconductor plates sandwiched together. Both plates are made of silicon and together, both are electrically neutral, with one side positive and the opposite side negative.
Atoms of phosphorus, element 15 on the Periodic Table, have 15 electrons and 15 protons respectively. Some atoms of phosphorus are added to the crystal silicon structure and take the place of silicon atoms. Since phosphorus has 5 valance electrons, four of the electrons bond with four silicon valence electrons leaving an extra electron. As more and more phosphorus atoms are exchanged for silicon atoms, an abundance of "free" electrons form. The "extra" electrons are available for bonding and this structure is now referred to as a negative plate or n-type plate with an electric field due to the "free" electrons. The positive layer of the photovoltaic cell is created in much the same manner.
Boron, element 5 on the Periodic Table, has 3 valance electrons and like phosphorus is exchanged for a silicon atom in the silicon crystal structure. When the boron atom is exchanged for the silicon atom in the structure, there are only 3 electrons available for bonding instead of four. This situation creates a "hole" because the missing electron leaves one "extra" proton in the structure. This layer is now called the positive plate or p-type layer. Because both plates are electrically neutral together, energy (extra electrons) must be added into the system in order to get electrons to flow (electricity) out of the n-plate and fill the "holes" in the p-plate. When placed in sunlight, photons which compose sun light, strike the photovoltaic cell and their energy can dislodge an electron from the n-plate to fill a "hole" in the p-plate, beginning a flow of electrons. Since silicon wafers are extremely reflective and somewhat fragile, a glass sheet or a cover plate or some other type of protective material is placed over the cell to reduce its reflective nature and protect the photovoltaic cell. Please keep in mind that this is a very simplified example of the composition and function of a photovoltaic cell and much more detailed information is easily accessible on the web or check in the Teacher Resource section of this unit. Single photovoltaic cells can be assembled together to form "modules" and modules can be assembled together to form "arrays." On a larger scale, the arrays can then be configured to meet almost any electrical need by connecting them in "series" or "parallel."
Solar Panels: Series and Parallel
A solar panel (module) is an assemblage of individual photovoltaic cells usually encased in glass and housed in a metal frame. You can easily construct a working solar panel by assembling individual photovoltaic cells purchased from a source such as Ebay® to meet most common DC electrical needs or convert the DC electricity into AC through the use of a converter. Suppose, as an example, you purchase 24 cells and when placed in direct sunlight, a single cell can provide 0.5 V at 3.6 A. To assemble these cells into a solar module (panel) you would begin by laying out the cells six in a column by four columns. By connecting all six cells in a column together in series you would have a column with an output of 3 V and 3.6 A because in series, voltage adds and current remains constant as outlined below using the power equation.
In a series circuit, when adding voltage the current will remain the same. Thus, for the six cells in series you have:
( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) = 3.0 V at 3.6A
The Power for this column of six cells would be
Power (W) = Current (A) X Voltage (V)
Six cells in series: Power (W) = (3.6 A) X (0.5 V X 6 cells) = 10.8 W
By continuing this process for all four columns you will now have four columns, each at 3.0 V and 3.6 A. The solar panel can then be brought to the "customary" 12 volts DC, simply by connecting these four columns together in series resulting in a 12 V module able to supply 3.6 A of current and a power output of 43.2 watts as outlined below.
Column 1:( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V)
= 3.0 V at 3.6A
Column 2:( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V)
= 3.0 V at 3.6A
Column 3:( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V)
= 3.0 V at 3.6A
Column 4:( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V) + ( 0.5 V)
= 3.0 V at 3.6A
For the Module: Column 1 (3.0 V) + Column 2 (3.0 V) + Column 3 (3.0 V) +
Column 4 (3.0 V) = 12.0 V at 3.6 A
Power (W) = Current (A) X Voltage (V)
Four columns in series: Power (W) = (3.6 A) X (3.0 V X 4 columns) = 43.2 W
If you need more watts, simply add panels together in parallel and construct an "array." As an example place four of the solar modules together in parallel where the voltage remains the same and the current increases as outlined below.
Power (W) = Current (A) X Voltage (V)
Four solar panels in parallel: Power (W) = (3.6 A X 4 panels) X (12.0 V) = 172.8 W
As you can see, by constructing your own solar modules and arranging them in series, parallel, or any combination you can generate the amount of electricity you will require for almost any need. With the inclusion of an inverter you can convert the DC electricity into AC. In fact, many home users of renewable solar generated electricity have a battery system to store electricity when the solar modules are not generating electricity due to cloudy conditions or night time. The same photovoltaic module system can be connected to a "power conditioner" which converts the DC electricity into AC (alternating current). The electricity can be used directly by the "load" (household use) and any excess electricity not being used can be sold to the local utility company.
The last component of a successful solar powered project is in understanding solar insolation. Solar insolation is the amount of sunlight in your area, which helps you to place your modules or a system at the best possible angle to collect the most sunlight and be as efficient as possible. NASA and the National Renewable Energy Laboratory have many maps and data sets available on line which will provide you with a good overview of the available sunlight and estimated kwh/m 2/day in your area. Keep in mind that these are average yearly totals and the summer will provide more sunlight while the winter will provide less. A final thought to consider in determining whether or not to switch to a renewable source such as photovoltaic cells is to consider that in California the average cost of a single kwh is approximately 15 cents which is comparable to the cost of a photovoltaic cell produced, single kwh. But, when you add in the financial incentives from California's renewable energy rebates, the photovoltaic cell generated, single kwh drops to as little as 8.9 cents over the 20 year lifetime of the system.

Comments: