Objectives
The IB objectives addressed in my curriculum unit include revisiting energy topics addressed in the first year of IB Physics, such as forms of energy and the laws of thermodynamic as they relate to creation of work in a cyclical process. Students will learn about and discuss energy degradation. They will construct and analysis energy flow diagrams and identify where the energy is degraded. Students will research and understand the different world energy sources comparing energy density, direct and indirect costs, environmental impact and advantages and disadvantages of each source of energy.
The common core objectives addressed in my curriculum unit are from standard five, matter and energy, where students understand the properties of energy in its various forms and the processes by which energy is exchanged and transformed. In addition, common core standard seven identifies and evaluates the uses of the Earth's resources. My curriculum unit will integrate all of these standards into a project where students will research and synthesize their knowledge about energy resources.
The Details of Energy
Energy is a crucial factor of our survival. We as humans cannot do anything without energy. If you are not convinced of this fact, try to not eat for a week and you will know what it is like to have no energy. Energy is defined as the ability to do work. So what is work? Work is using a force to move a mass though a distance. It is very much like lifting a book to a table or walking around the block. The great news about energy is that energy is conserved. It is a law of nature, specifically, the first law of thermodynamics. It states that energy is neither created nor destroyed but changed from one form to another. Here, however, is the bad news. The second law of thermodynamics, which states that energy always goes from a more useful form to a less useful form, means that energy is constantly changing forms to something that cannot be used to do work.
Energy is grouped into three categories: kinetic energy, potential energy, and rest energy. Kinetic energy is energy due to a mass moving. Anything that moves has kinetic energy from the car speeding on a highway to the molecules moving in your hot coffee, in addition to electrons moving a current in a circuit. Kinetic energy is also in sound waves and electromagnetic waves of light. All this kinetic energy is doing work, moving a mass through a distance. Work is done in moving a car and in moving an electron in a circuit.
Potential energy is stored energy in a field. For example, a compressed spring or water behind a dam can be used to do work later. Potential energy is stored in the chemical bonds of fossil fuels and in electric fields of capacitors.
Rest energy is the energy stored in the nucleus of the atom. When two or more protons form the nucleus of an atom, some of the proton's mass is converted to energy. This energy is called the binding energy and is found using Einstein's famous E=mc 2 formula. The Sun's energy is derived from the binding of hydrogen in nuclear fusion to form helium and, as a result, energy is released during this reaction. The energy released from the sun is in the form of electromagnetic waves or light, which the earth receives. We use the binding energy in nuclear reactors to create heat and electrical energy in nuclear power plants.
The Laws of Thermodynamics
The conservation of energy is the most widely applicable law of nature. It is the governing principle from supernova to the operations of the cells in your body. When I first introduce the concept of energy, I discuss the first law of thermodynamics, it states that energy is neither created nor destroyed but changed from one form to another, and use a roller coaster as an example. We use a motor to move a car to the top of the first hill and then release the car. The gravitational potential energy stored in the car propels the car to the bottom of the hill at great speed and then back to the top of the next hill slowing down as the kinetic energy is converted back to gravitational potential energy. If there is no friction between the wheels of the car and the track, the car will return to the same height on the second hill. However, if there is friction, some of the energy is converted to heat. The track will get hotter and the wheels of the car will also increase in temperature. Energy is conserved but some of the energy is converted to heat and eventually the roller coaster car will come to a stop.
The second law of thermodynamics restricts the conversion of heat energy to useful work and there are multiple ways to state the second law. One way to state this is heat energy always flows from hot (high energy) to cold (lower energy). Again, the concept of the second law of thermodynamics is very simplistic but the implications are much more complicated. For example, to make something colder than its surroundings, work must be done on the system (energy input). The next part of the second law states that it is impossible to build an engine or cycle that only converts heat into an equivalent amount of work. This means that NOTHING is 100% efficient. In all heat cycles, some energy is dissipated to the environment as waste heat.
The result of the second law is high energy heat sources deposit heat to lower temperature reservoirs, then heat from these lower temperature reservoirs deposit heat to still lower temperature reservoirs until the accumulated waste heat ends up in the environment. For example, if coal is burned as a source of electrical energy, only about one-third of the energy in the coal can be transmitted to our homes as electrical energy; the other two-thirds goes to the atmosphere as waste heat. 2 Modern electrical generators that convert mechanical energy into electrical energy are within a few percent of the maximum efficiency dictated by the second law of thermodynamics. Ultimately, the second law of thermodynamic tells us we cannot get something from nothing.
Sources of Energy and Energy density
At the end of the last century more than ninety percent of all energy used by humans originated from fossil fuels. 3 Why do we love Fossil fuel? Fossil fuels pollute the atmosphere with carbon dioxide and other pollutant that cause acid rain. We have to dig them from deep within the earth, which destroys the landscape and creates other forms of pollution. It is because of fossil fuel's potential energy. Fossil fuel comes from the sunlight falling on the earth millions of years ago being converted and stored in chemical energy of the cell of plants and animals. So how does fossil fuel compare to other energy alternatives. To make a comparison we need to have a common unit for all energy sources, and that common unit is energy density of the energy per kilogram. If we look at the energy in one pound of fat, it has about 3600 Calories of energy or about 15 million Joules of energy per pound. That is why it takes so much work to burn off an extra pound of fat. If we convert the pound to kilogram there is about 33 million joules of energy per kilogram of fat. Gasoline has about 46 million joules of energy per kilogram a factor of 1.4 times greater. Below is a chart of energy density of common sources of energy in mega (million) joules per kilogram. The values listed are approximate due to variation in the chemical composition. 4
Table 1.1 Energy Density in MJ/kg
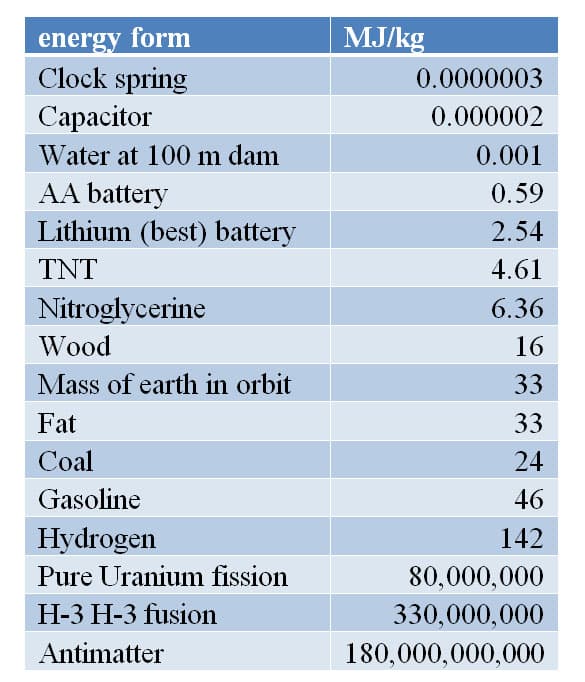
The most striking values on this table are the energy stored in the nucleus of atoms and antimatter. It is clear why when the idea of nuclear fission became an option as a source of energy it was stated "too cheap to meter." 5 Nuclear fusion and antimatter are not possible sources of energy today but could be future sources. The only chemical energy source available with greater energy per kilogram than gasoline is hydrogen. There are two problems with using hydrogen as a fuel source. First, there is not a source of pure hydrogen we must separate it from water or other hydrogen containing compounds, and which takes energy to separate. Secondly, hydrogen is a gas at room temperature and one kilogram of hydrogen will have four times the volume of one kilogram of gasoline so, hydrogen has 4.5 times less energy than a gallon gasoline and 30-40% of the energy available is used in producing the pure hydrogen. 6 We love gasoline because is has a lot of energy per kilogram.
Notice solar, wind and hydro energy are not listed on the table. That is because they are not chemically stored sources of energy. They are power sources. Power is the rate at which energy is produced or used, measured in kilowatts or kilojoules per second. The average American home uses about one kilowatt (or 1000 joules per second) of power. 7 The sun provides approximately one kilowatt of energy per square meter when it is directly overhead. That is enough to power the average America home and is equivalent to one gigawatt per square kilometer enough power for a million homes. Except, solar cells at best are only about 15% efficient in converting the electro-magnetic energy of the sun into the electrical energy we desire. The area to produce one gigawatt of power is about 50 square miles. 8 In addition, power from solar energy would still have to be stored in batteries for use during cloudy days or at night, which is an additional cost and efficiency issue.
Wind energy is a valid alternative energy source in selected regions around the world. A wind farm of 130 wind turbine generators has been proposed on the ocean off the coast of Massachusetts to supply commercial power. The projected area used is 28 square miles and it will produce a maximum of 0.43 gigawatts of power and a yearly average of 1.5 gigawatt-hours. 9 Other alternative such as geothermal, wave and tidal sources are possible in selected regions, but the ability to produce significant amount of power in the near future is limited.
In Table 1.2 the approximate energy cost for various power sources are listed in kilowatt-hours. Notice the cost for fossil fuels are all greater then the cleaner alternatives such as nuclear, hydro and wind. What is prominent on this table is the cost of batteries, which needs to be addressed to students because, generally, they have no clue as the true cost to buy batteries even, rechargeable cell phone batteries.
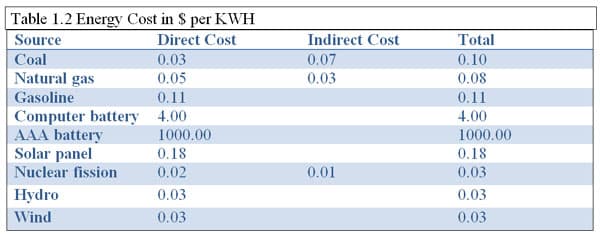
Table Notes: Direct cost includes capital cost, operation, maintenance and fuel costs. Indirect costs include social and environmental costs. Indirect costs not listed have not been qualified. 10
Energy Alternatives
The first student assignment will be to develop a class presentation of energy sources and alternatives. Each student or group of students will be assigned a source of energy from the following: fossil fuels, nuclear, solar, wind, hydro, which includes wave and tidal. The students will prepare a class presentation on how their particular source produces power, cost per kilowatt-hour, efficiency in the form of a sankey diagram. A sankey diagram is a graphic that illustrates the flow of material or energy where the width of the arrow is proportional to the magnitude. In addition to a description of the power provided by the source of energy, environmental concerns and a direct comparison of advantages and disadvantages must be presented and discussed. The students will make 15-minute presentation to the class on the energy alternatives and a scoring rubric for the student's presentation is in Appendix 3.
World Energy and Energy for the Future
Described above are the science and facts about energy, the energy density and the costs of the different energy resources. My students will make presentations on the different energy sources available today, but what will we use in the future and how will it impact our climate and economy worldwide is the real question. We love fossil fuels because they have lots of stored energy and are efficient to use. We will exhaust the supply of oil reserves, but coal will last for centuries. Coal is environmentally the worst source of energy. 11 Is nuclear energy a valid alternative or is solar and wind the best alternative? How will our energy needs and sources develop in the future? I want my students to research world energy uses and supplies and develop an energy policy that meets energy needs for one region of the world for the next 25 years.

Comments: