One of the things I love most about being a teacher is that each day I get to see the direct impact of the work I do. Over the past five years, I have seen just how high the stakes are for my students. But every skill or life-lesson I teach them can open the door for opportunities and put them on the life path to becoming engaged global citizens in an every changing society. This curriculum unit will allow my students to discover where the energy in fossil fuels actually comes from. Students will engage in a series of problem-based learning experiences in order to connect several large concepts including: the chemistry of photosynthesis, the role photosynthesis played in the evolution of Earth’s atmosphere, fossil fuel combustion, and atmospheric chemistry.
The New Haven 7th grade curriculum includes content aligned with the Physical Science and Physical Chemistry seminar topic. This seminar will connect with the second unit in the 7th grade curriculum (Chemical Properties) including: chemical equations. 10
This curriculum unit will focus on the chemistry behind photosynthesis. Students will use the chemical equation for photosynthesis to learn about properties of atoms vs. compounds, reactants and products in a chemical equation, balancing chemical equations, and subscripts and coefficients. On a deeper level my students will be able to discover where the energy in fossil fuels actually comes from by examining the reverse reaction of photosynthesis, combustion.
This chemical equation will also be used to analyze the role photosynthesis played in the evolution of the Earth’s atmosphere. Students use problem solving skills to analyze data from air samples collected from amber. The Keeling curve will also be used to compare oxygen and carbon dioxide trends over the course of seasons and years.
A major focus of our unit will be on oxygen production and carbon dioxide uptake in photosynthesis and carbon dioxide release in fossil fuel combustion. I would then like my students to create a culminating project in which they create a 3-D model or diagram to compare the chemical equations for photosynthesis and fossil fuel combustion.
I believe creating a curriculum unit focused on energy sciences, will allow me to change the minds of my students and create citizens capable of making informed decisions about energy use. This unit will foster transformational change by impacting all areas of my students’ lives, from academic growth to problem solving and career development. Academically, my students will gain exposure to real world scientific connections. They will engage in cutting-edge work of the discipline, through hands-on activities and demonstrations. This unit will also focus on problem solving. My students, like scientists, will practice thinking critically and creatively to solve problems that relate to the world around them and other fields of science and mathematics. And finally, this unit will provide my students with a deeper understanding of STEM careers and hopefully spark their interest in pursuing a degree in science, technology, engineering, or mathematics to discover potential future sources of energy.
Energy for Ecosystems
An ecosystem is made up of all the living and nonliving things that interact with each other in a certain place. The living things in an ecosystem depend on one another, but they also rely on nonliving things around them. 14 Within ecosystems, there are interactions between the living things and the nonliving environmental features, such as water, temperature, sunlight, soil and air. The abiotic factors impact the living organisms; for example, the temperature and availability of water affect plant and animal growth. The living organisms impact the environment; for example, plants release oxygen to the air and materials from decomposing leaves enrich the soil. 14 A vital nonliving thing, the sun, is the source of food in some way or another for all living things. 14
All living things require energy and matter in order to maintain order, grow, and reproduce3. This energy is cycled through ecosystems and is passed from one living thing to another. 14 Organisms rely on a variety of evolutionary strategies to capture, use, and store energy. But the source of all this energy is sunlight. The sun is the main source of energy for all life on Earth. For example, autotrophs capture energy from their environment through photosynthesis and chemosynthesis. 14 Plants, algae, and some bacteria use the energy from the sunlight to make their own food. This food is the source of energy for all living things in an ecosystem. For example, herbivores gain the energy they need to survive by eating plants.
Earth’s Cycles5
The world we live in is constantly changing, but many natural phenomena make cycles, allowing them to come back to the way they were. Some important cycles include the water cycle, oxygen cycle, carbon cycle, nitrogen cycle, and phosphorus cycle. 5 Although these cycles are natural and ongoing, human activities, such as agriculture, industry, and energy production, can disrupt the flow of the Earth’s cycles. For example, burning fossil fuels, such as coal, oil and natural gas, affects the carbon cycle. 5 To combat these ongoing problems and work toward a “greener” world we need to look for ways to go “green.” 5
Photosynthesis
The process by which plants use energy from the sun to make their own food is called photosynthesis. 16 In multicellular plants, photosynthesis occurs in tiny structures called chloroplasts in the cells. 16 In most plants, chloroplasts are found in the leaves. Chloroplasts contain a green pigment called chlorophyll that absorbs the energy from the sunlight. 14 It is this pigment that gives leaves and stems their green color. The absorbed energy from the sun is used to change carbon dioxide and water into glucose (sugar) and oxygen. 16
Photosynthesis occurs in a series of “enzyme-mediated steps” that allow the plant to capture light energy from the sun and use it to build energy-rich carbohydrates. 3 The process is summarized in the following unbalanced chemical reaction:
CO2 + H2O + Sunlight -> C6H12O6 + O2 (1)

Figure 1. Photosynthesis in plants. 1 (At09kg, 2011. Copyright free licensing under Wikimedia Commons.)
The sun is the main source of energy on Earth and it plays a vital role in photosynthesis. During photosynthesis, carbon dioxide (CO2) and water (H2O) join chemically to produce oxygen (O2) and glucose (C6H12O6), which is an energy-rich sugar.14 Green plants use light energy to make sugar molecules from the atoms of water (H2O) and carbon dioxide (CO2). 6 During this chemical change, green plants release oxygen needed by most organisms for respiration. Plants and animals take in oxygen and use it to release the energy stored in sugars and other molecules produced during the photosynthesis. 6 During this respiration process, CO2 is released into the air or water.14
Plants’ ability to do photosynthesis is affected by atmospheric conditions such as precipitation amount and air temperature. For example, plants absorb water from the soil using their roots. Soil is a mixture of materials that includes weathered rocks and decomposed organic material, as well as air and water. 14 The composition of soils affects how air and water move through the soil, and this influences the kinds of plants that can grow and the animals that rely on those plants for food. Carbon dioxide is another reactant in this reaction and this gas is taken in through small openings in the leaves of plants called stomata. 14 Once inside the leaves, water and carbon dioxide move to the chloroplasts. A series of chemical reactions lead to the production of glucose and oxygen. 14 Plants use this energy-rich sugar, glucose, for food and release the oxygen into the air as waste.
Energy Behind Photosynthesis
Energy is a property of everything. Energy must be put into something in order to get energy out. In the example of photosynthesis, solar energy (light) must be put in order to get chemical energy out. This process involves taking low-energy chemicals and converting them into high-energy chemicals. The reactant side to the photosynthesis equation is: CO2 + H2O + Sunlight. 6 CO2 and H2O have strong bonds with low energy. While the products side of the chemical equation is: C6H12O6 + O2. C6H12O6 and O2 have weak bonds with high energy.
Why is light required?
Since the reactants side is made up of strong bonds, energy is needed in order to break these strong bonds. Sunlight is the natural energy source for photosynthesis.
Light is an electromagnetic wave. The light we see is called visible light. 9 This light is only a small part of the electromagnetic spectrum. White light from the sun is a mixture of all colors of the light spectrum. Visible light, or white light, can thus be separated into individual colors or light. 9 Visible light is seen as red, orange, yellow, green, blue, indigo, and violet. Each color has a unique wavelength. Red light has a long wavelength, but the lowest energy of all visible light. 9 Violet on the other hand has the shortest wavelength of all visible light, but the highest energy. 9
Light controls many plant processes. Light can either be absorbed or reflected by substances called pigments. Most plants are green because the pigment chlorophyll reflects green and yellow light and absorbs the other colors of the spectrum. Chlorophyll in plants absorbs mostly blue and red wavelengths of light. Blue wavelengths, 400-450 nm, and red wavelengths, 625-700 nm, are necessary for photosynthesis. 9 Photoperiod responses, the physiological reaction of plants and animals to the length of day or night are also triggered by red wavelengths. 9 Phototropic responses, the growth of plants toward a light source, are triggered by blue wavelengths.9 The average rate of energy capture by photosynthesis around the world is 130 terawatts. 15 This is about seven times the current power consumption of the planet. Photosynthetic organisms are able to convert around 100-115 thousand million metric tons of carbon into biomass each year. 15
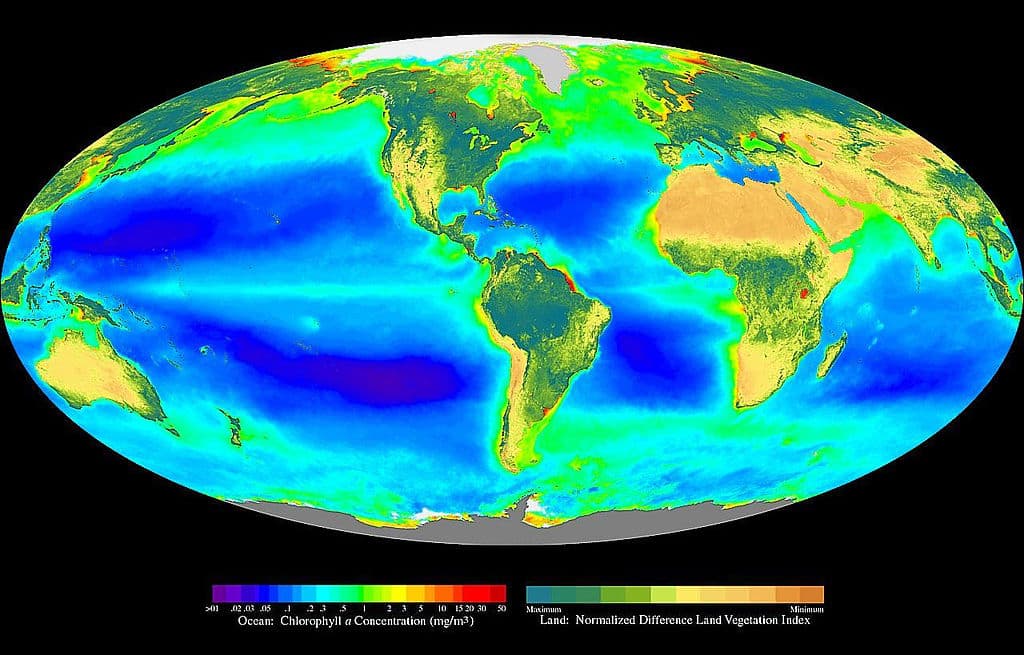
Figure 2. Composite image showing the global distribution of photosynthesis, including both oceanic phytoplankton and terrestrial vegetation. Dark red and blue-green indicate regions of high photosynthetic activity in ocean and land, respectively. 13 (SeaWiFS Project, 2005. Copyright free licensing under Wikimedia Commons.)
As shown in the figure above, plants and algae dominate the biosphere. 13 Inside the cells on the leaves of plants are special structures called chloroplasts. These chloroplasts contain layers of membranes and used to be free-living organisms. 13 Scientists believed these organisms were cyanobacteria and data show that they have their own DNA and mechanisms for making proteins.
Process of Photosynthesis
In this light-dependent reaction, some energy is used to remove electrons from water to produce oxygen gas. The hydrogen ions and electrons freed when water is split are then use to create two other compounds, nicotinamide adenine dinucleotide phosphate (NADPH) and adenosine triphosphate (ATP), the energy of cells. 6 There are five main complexes involved in photosynthesis: photosystem II (PSII), cytochrome, photosystem I (PSI), ATP (energy), and NADPH (provides electrons). 2 PSII and PSI are the reaction center complexes that are specifically involved with absorbing light and transferring electrons through the electron-transport chain. Both PSII and PSI contain chlorophyll but they absorb different kinds of light. PSII and PSI both absorb blue light, but PSI absorbs more red light.6 These photosystems receive light excitation energy from light-harvesting complexes, associated with their reaction centers. The photosystem is also where charge separation occurs.
When light is absorbed by the chlorophyll in the photosystem it creates an excited state. This excited state allows the donor to give an electron to the accepting molecule. This donation of an election to the accepting molecule is called charge separation. 6 Plants are able to create a stable charge separation, so recombination doesn't occur and this energy can be stored.
Light-dependent reactions
In the light-dependent reactions, one molecule of chlorophyll absorbs one photon of light and loses one electron. As mentioned above this electron is donated. It is passed to another form of chlorophyll called pheophytin. 6 This passing of electrons begins to create a flow of electrons known as the electron-transport chain. This has two affects on the plant cell. First, it creates a reduction of NADP+ to NADPH. Secondly, it also creates a proton flow that is necessary for the production of ATP. 2 The chlorophyll molecule is able to regain the electron it lost when water is split and an O2 molecule is released.
Electrons generated in PSII by water splitting are passed along the electron-transport chain to PSI where another excited state is created when light is absorbed. 2 The electrons passed along the electron-transport chain are used to reduce NADP+ to NADPH, as shown on the Z scheme diagram below. 17

Figure 3. The Z-scheme. 17 (w:User:Bensaccount, 2005. Copyright free licensing under Wikimedia Commons.)
Chemical Equations
Molecules are identified by chemical formulas. 14 A chemical formula is a group of chemical symbols and numbers that show the kinds and number of atoms in a molecule.14 For example, the formula for water is H2O. H is the chemical symbol for hydrogen. O is the chemical symbol for oxygen. The small number 2 is called a subscript. It shows that the molecule contains two atoms of hydrogen. The O has no subscript, which means that the molecule contains only one atom of oxygen. (Note: It is not necessary to write 1 as a subscript in chemical formulas or equation. No subscript recognizes there is only one atom.)
Balancing Chemical Equations
In a balanced chemical equation, certain conditions must be met. A chemical reaction is simply the rearrangement of atoms to form something with a new chemical formula and different properties. 14 Balanced chemical equations involve an equal number of atoms on both the product and the reactant sides of the equation. 14 In the unbalanced photosynthesis equation (1) shown above there are 1 C, 3 O, and 2 H on the reactant side and 6 C, 12 H, and 8 O on the product side. The following numbers need to be added in order to make both sides of the equation have 6 C, 18 O, and 12 H.
6 CO2 + 6 H2O + Sunlight -> C6H12O6 + 6 O2 (2)
Keeling Curve 12
The Keeling Curve (Figure 4) is a graph that plots ongoing changes in the concentration of carbon dioxide in Earth's atmosphere.4 Continuous measurements have been taken since 1958 from the Mauna Loa Observatory in Hawaii, which originally began under the supervision of Charles David Keeling. 12
The Keeling Curve shows a “saw-tooth” pattern, with CO2 levels typically falling from May through September and rising over the rest of the year.12 This cycle is due to the natural exchanges of CO2 from plants. 12 Despite this “saw-tooth” pattern, CO2 levels have continued to rise higher than the year before due to the burning of fossil fuels.12 According to data from Mauna Loa, CO2 levels have increased about 2.2 ppm per year.12

Figure 4. Atmospheric carbon dioxide concentrations from 1958-2015. 4 (Delorme, 2015. Copyright free licensing under Wikimedia Commons. Based on data from Dr. Pieter Tans, NOAA/ESRL and Dr. Ralph Keeling, Scripps Institution of Oceanography.)

Comments: