Appendix C: Dye Sensitized Solar Cell Lab
Materials
Conductive FTO glass *
Nanocrystalline TiO2 powder *
Potassium Iodide Electrolyte solution*
Graphite pencil *
Binder Clips *
Leaves from plants (chlorophyll dye)
Dilute acetic acid (0.1mL diluted in 50 mL deionized water)
Mortar and pestle
Multimeter
Dishwashing soap
Ethanol
Alligator clips
Hotplate
Transparent tape
Water wash bottle
Berries (Black, Blue, Raspberries)
Materials with an asterisk are available in the Nanocrystalline Solar Cell kit from the Institute for Chemical Education: http://ice.chem.wisc.edu/Catalog/SciKits.html
Preparation of TiO2 Semiconducting paste
- Weigh 6 g of TiO2 powder into a mortar
- Add powder to 9 mL of dilute acetic acid (~ pH 2.5)
- Slowly add the acid in 1 mL increments to the powder while grinding with a pestle.
- Make certain a uniform suspension forms before adding additional acid.
- Continue adding acid in 1 ml increments, grinding carefully until a lump free suspension is formed (approximately 30 minutes). Resulting suspension should have the consistency of paint.
- Once finished add a drop of dishwashing liquid to the suspension: Do not grind after the soap is added. Transfer the paste to a syringe: cover end with paraffin to prevent drying out.
Preparation of Conductive Electrode
- Determine the conductive side of the glass using a multimeter. The conductive side will register resistance between 25 and 30 ohms.
- Use transparent tape to tape the glass to a clean sturdy surface. Use the tape to make a border around 3 sides of the glass: two sides should have a border of 1 mm, the top of the glass will have a 4 mm border (this is where the alligator clips will be attached): the fourth side has no border. The pieces of tape should help secure the glass to the work surface. Carefully clean the glass with a few drops of ethanol
- Place a small amount of TiO2 paste on the glass and immediately spread the paste with a glass rod to produce a thin uniform coating. Allow the electrode to dry.
- Once dry place the electrodes on a cold hot plate and set it to high. This will sinter the electrode. The electrode will turn brown then back to white. The process is complete after ~ 30 minutes.
- Turn the hot plate off and allow the electrodes to cool fully before handling.
- Use a second conductive glass to prepare the counter electrode. Find the conductive side; then use a graphite pencil to make a uniform coating of graphite on the entire glass.
Making the DSSC cell
- Prepare the dye by crushing a few berries and ~ 2 mL of deionized water in a small plastic bag.
- Carefully remove the tape from the white electrode, then place it in the bag with the berries. Wait for the dye to take effect.
- Remove the electrode with a pair of tweezers: wash carefully with water then ethanol. Let the electrode dry.
- Repeat this procedure with the dyes from other berries: when all electrodes are dry proceed to the next step.
Measuring Voltage
1. Using alligator clips, connect a multimeter to the edges of the electrodes: (attach the the negative (black wire) of the multimeter to the TiO2 electrode): the positive (red wire) to the counter electrode.
Figure 3: Dye Sensitized Solar Cell:
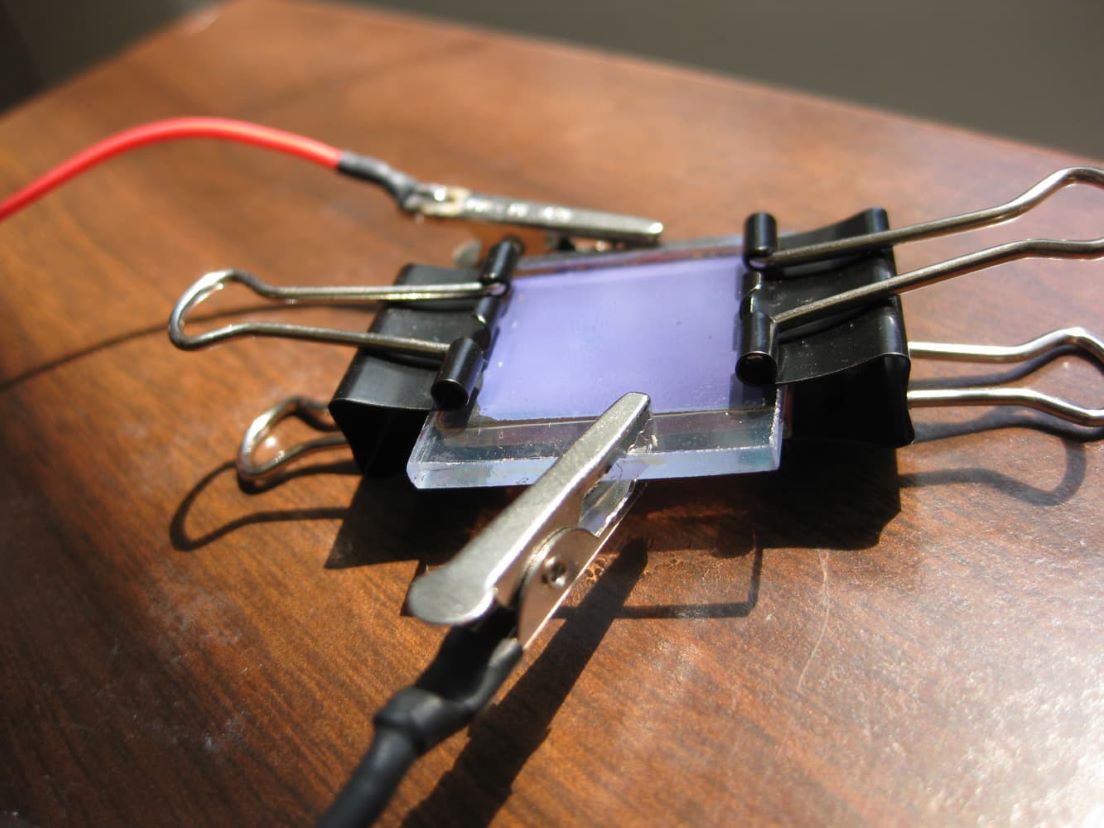
Source: http://www.nisenet.org/sites/default/files/images/catalog/5553/dssc.jpg
Used by permission of University of Madison Wisconsin MSREC Education Group: http://education.mrsec.wisc.edu/289.htm
2. Shine a light source on the cell then measure and record the voltage of your cell.
3. Compare the voltage of your cell with others in your group.
Once finished complete the analysis questions
Extension Activities
- Connect cells in series to power small electronic devices.
- Use chlorophyll extracted from leaves as a dye.
- What would happen if colored rather than white light were used as an energy source?

Comments: