Content Objectives
This unit is broken into three key sections. The first section is on the basics of coal, including how it forms, its distribution in the United States and worldwide, an overview of how it is mined, and how coal is used to generate electricity. The next section covers the specifics of mountaintop removal mining, and last section covers air pollutants and water pollution associated with coal mining and combustion. The latter two sections represent the majority of the content presented in this unit, as I cover natural resource extraction more generally in a separate unit.
Coal Basics
Coal Formation
Coal has its origins some 300 million years ago in the Carboniferous Period.5 During this time, the placement of the continental land masses, comparatively high atmospheric carbon dioxide (CO2) concentrations, and a warm and wet climate promoted an explosion in the diversity and size of plant life. These conditions can be likened to the tropical and subtropical humid climates found in the tropics today.6 Large sections of the earth were covered in swamp forests, where large plants captured sunlight and CO2 and converted it into biomass as they grew. Because this process begins with photosynthesis, coal (and indeed most fossil fuels) has been called ancient sunlight. When those plants died, the organic material in the plants underwent partial decay but still held a great deal of organic content. Over time, rivers and streams deposited sediment rich in this partially decayed organic material.7 As the climate continued to change, these organic rich layers were buried with inorganic sediments. This material underwent a series of transformations to become peat, an accumulation of partially decayed organic matter.8 The lignin and cellulose contained in the organic sediment are of specific importance to the formation of peat because they are the toughest parts of plant material and resist decay under normal conditions.9 However, the anoxic conditions of the shallow basin promoted the decay of these compounds in such a fashion that the nitrogen and oxygen were lost but the carbon preserved.10 Under specific temperature and pressure conditions, peat can undergo coalification. These conditions were present due to the burial by additional sediment, the overlying water in the basin, and the subsidence of the underlying crust. The first type of coal that emerges from these conditions is called lignite, a low grade, high-sulfur content coal. Additional heat and pressure continue to alter the chemical structure and eventually produce the next two type of coal, bituminous and anthracite. As the coalification process continues the energy content of the resultant coal increases, while impurities such as sulfur decrease. For this reason, anthracite coal is the most prized type of coal.11 One of the many reasons the Great London Smog had such a negative impact on human health was the burning of low-grade lignite coal with significant sulfur impurities.
Coal deposits in the lower 48 states are shown in Figure 2. Of specific interest to this unit are the bituminous deposits in West Virginia due to the environmental implications of the mining methods used to extract it. As the figure demonstrates, most of the coal in this region is medium volatile bituminous coal, which has more energy content and produces less sulfur when combusted than the lignite, subbituminous, and low volatile bituminous that dominates much of the available coal in the U.S.

Figure 2: Coal deposits in the lower 48 states.12 The dark grey and grey-blue deposits are medium and high volatile bituminous coal. The red deposits are low volatile bituminous coal. The two shades of yellow are lignite coal. The two shades of green are subbituminous coal. Most of the active coal mining operations in the U.S. are in medium volatile bituminous deposits (Appalachia) and in subbituminous deposits (Wyoming).
Extracting Coal
There are several different methods of mining coal, categorized as either surface or subsurface methods. Surface methods include contour mining, strip mining, and MTR. Subsurface methods include room-and-pillar mining and longwall mining.13,14 Students learn about various mining methods in a separate unit of study on natural resource extraction, but present a general review of coal-specific mining techniques. In the various surface mining techniques, the procedure is essentially the same: removal of topsoil, drilling and blasting overlying strata, moving this material (called spoils), drilling and blasting the coal seam with explosives, removing the coal and transporting it away, backfilling with spoils, and reclaiming the area with soil and vegetation.15 The specific technique is chosen based on the geography and geology of the region. In particularly hilly terrain such as the Appalachian region of the U.S., contour mining or MTR is used since there is limited space to transport and store overburden. In the Midwestern region of the U.S. traditional strip-mining techniques are used. Subsurface mining is also dictated by the geology of the coal seam and surrounding area. Ultimately the thickness depth and angle of the seam, combined with the amount of gas in the subsurface dictate whether the coal is extracted using room-and-pillar or longwall techniques. In room-and-pillar mining, large “rooms” are created as coal is extracted. “Pillars” of coal may be left in place to hold open the space and engineered supports are added to prevent collapse of the overlying strata. Longwall mining involves the cutting of long stretches of rockface and extracting the coal, leaving no support pillars in the area. As mining advances the roof of the previously mined section is allowed to fall.16
Surface techniques are most economical and effective for relatively shallow deposits, while subsurface mines are often the better choice for deeper deposits. In some cases, a combination of the methods is used. In general, surface mining has advantages over subsurface mining, including higher recovery rates, health and safety statistics, and lower amounts of pre-combustion processing. Because of these factors, surface mining tends to be significantly cheaper than subsurface mining.17 Mountaintop removal mining is discussed in more detail below as it is a central focus of this unit.
Producing Electricity from Coal
It is critical to understand that the coal that comes out of the ground isn’t a direct source of electricity. I can’t power my laptop by plugging it into a chunk of coal. The production of electricity from coal involves the combustion of pulverized coal in order to produce steam from water. This steam then turns a turbine which turns a generator. As the name implies, this device generates electricity. The electricity is then transported along the electrical grid. This is demonstrated in Figure 3. The grid is a network of interconnected transmission lines that distributes electricity from power plants or other electricity-generating sources to users of electricity such as homes or businesses.18
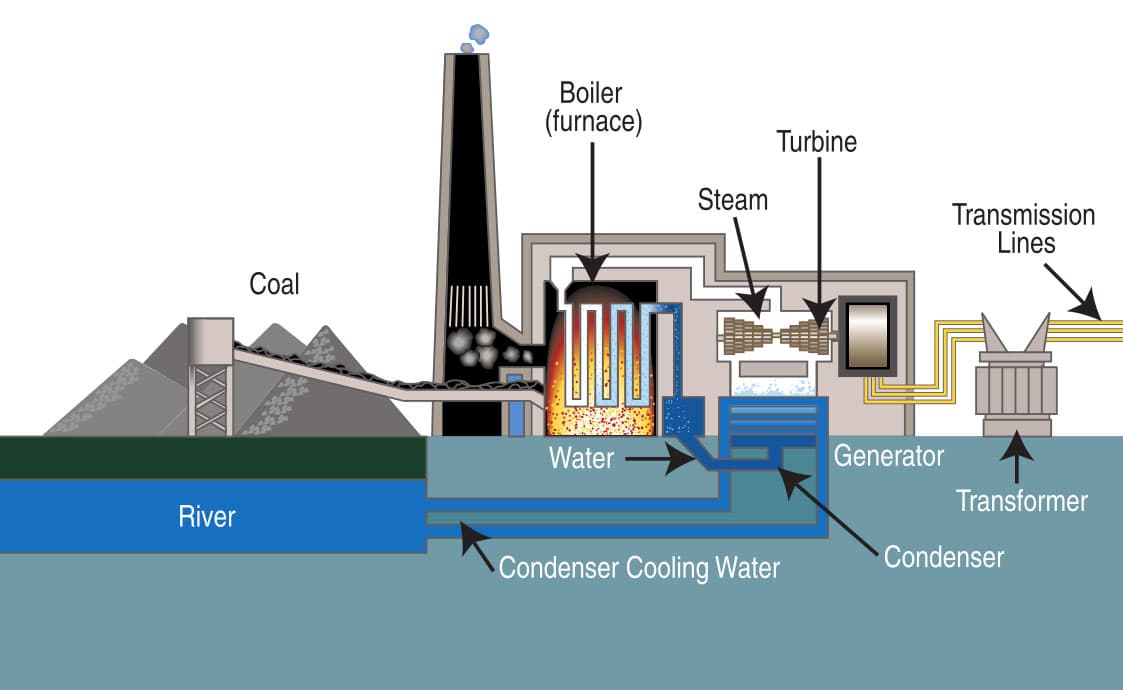
Figure 3: Diagram of how a coal-fired power plant generates electricity.19
As simple as it sounds there are several issues with using coal as a source of electricity. First, the coal must be mined, refined, and transported to the power plant. Second, the global average efficiency rate of coal-fired power plants is between 30 and 40 percent. This means that if 100 units of coal are burned in a power plant, only 30-40 of those units get converted into electricity. The remaining units are converted to waste heat.20 Finally, there are several pollutants that originate from coal combustion, including mercury, sulfur, and CO2.21 Mercury and sulfur emissions are addressed below, while CO2 is covered in a separate part of my course that specifically focuses on the causes, mechanisms, and impacts of anthropogenic climate change.
Mountaintop Removal Mining
Mountaintop removal mining (MTR) is of particular interest given the proximity of such mining operations in Appalachia. I use the analogy of a layer cake when discussing MTR with students: just as in a layer cake there are alternating layers of frosting and cake, there are mountains with a layer of rock, layer of coal, layer of rock, layer of coal, etc. To get at the layer of coal, MTR removes the overlying rock using explosives. The layer of coal is then extracted and the process is repeated until the coal layers are exhausted. The rock removed during the process, called the overburden, is typically dumped into valleys between mountains. When the mining operation is complete, this material is sometimes replaced on what is left of the mountain. In other situations where valley fill is not possible, the overburden is hauled away. I present the environmental hazards associated with MTR later in this unit. MTR is favored by the mining industry because it involves less manpower and financial risk compared to traditional strip and subsurface mining techniques.22 MTR became the primary method of coal mining in Appalachia in the 1990s as the industry needed a way to cheaply extract the low-sulfur coal located in the region. This shift was brought about by stricter EPA regulations on sulfur emissions aimed at reducing sulfur dioxide emissions and associated acid deposition after decades of environmental degradation associated with the burning of low-grade coal.23
Environmental Impacts
The environmental impacts of MTR are quite dramatic. In fact, these mining operations can be seen on satellite images due to their scale and dramatic impacts on land cover (Figure 4). What was once covered by dense forest ends up being bare rock.

Figure 4. a) Satellite view of Central West Virginia. b) Zoomed in view of MTR sites (upper left and right corners and center bottom). c) View of a single MTR operation. Individual images courtesy of Google Maps.
The major environmental impacts of MTR include deforestation and habitat loss (as seen in Figure 4), burial of stream headwaters by overburden, leaching of hazardous material into ground and surface water, and release of particulate matter and sulfur species into the atmosphere.24 The burial of stream headwaters presents several environmental challenges, including alterations to surface and subsurface flow, habitat loss, changes to downstream channel morphology, and reductions in stream biodiversity.25 It has been reported that 90% of streams below valley fill MTR operations were “impaired” by Clean Water Act standards. Specific impacts include a reduction in macroinvertebrate species and number of individuals, disappearance of fish species, and reductions in salamander species. The use of explosives to remove the overburden and mine the coal seams releases a great deal of particulate matter into the atmosphere, which can cause upper respiratory illness and, in some cases, can lead to cancer.26 Sulfur species can enter the atmosphere when sulfate from mining runoff is reduced by bacterial species into hydrogen sulfide gas. Sulfate aerosols from the mining operation are also released into the atmosphere.27 Particulate matter and sulfur pollution are discussed in more detail below since they are not exclusively associated with MTR, but with coal mining and combustion more generally.
Particulate Matter
The combustion of coal is a leading source of toxic air pollution globally.28 Particulate matter (PM) is a major component of coal pollution, and is a complex mix of fine particles and liquid droplets and may include acids, organic chemicals, metals, and soil or dust particles.29 PM originates in two ways: the first is direct emission of particles from combustion reactions, construction sites, unpaved roads, etc. The second is through atmospheric reactions of primary pollutants such as SO2, and nitrogen oxides (NOx) that are emitted from power plants, industrial operations, or from automobiles.30 In terms of PM related to coal, both types of PM are emitted. A great deal of primary PM is emitted in the mining process through blasting of rock and transportation of materials to and from mines on unpaved roads. The composition of this PM is distinct from secondary PM, and is mostly dust, silica, and elemental carbon. Secondary PM is also produced as coal-fired power plants emit SO2 that reacts with atmospheric constituents. As discussed above, some of this material settles out of the atmosphere through dry deposition and some goes on to become acid rain.31
Health Impacts
There are several categories of PM based on its aerodynamic equivalent diameter (AED). Typical size categories are less than 10 µm, 2.5 µm, and 0.1 µm (PM10, PM2.5, and PM0.1). PM with an AED greater than 10 µm has the shortest suspension half-life and will typically be filtered out by the nose and upper respiratory system. The smaller particles have longer suspension half-lives are not as easily filtered by the human respiratory system. The ultrafine particles pose a more significant health threat even though they represent a much smaller fraction of the total mass of PM pollution.32
Several studies have shown that the specific impacts of PM pollution vary by AED, but in general such impacts include cardiovascular, cerebrovascular, and respiratory diseases.33 There is also strong emerging evidence for PM-related lung cancer, as evidenced by the declaration of PM as a Group 1 carcinogen by the International Agency for Research on Cancer.34 Impacts on the cardiovascular system are thought to be related to inflammation brought about by PM exposure. There are also links to problems with changes to coagulation of blood and platelet activation. In general, there are strong links between PM exposure and ischemic heart disease, congestive heart failure, and acute myocardial infarction.35 Exposure to PM has also been linked with cerebrovascular health effects. The mechanisms, risk factors, and features for PM-related ischemic cerebrovascular disease are similar to those for cardiovascular disease. However, the specific links to PM-related strokes and other cerebrovascular diseases are less well understood than with PM and cardiovascular disease.36
It should come as no surprise that exposure to PM can lead to respiratory illness, especially for the PM2.5 and PM0.1. However even PM10 can lead to or exacerbate existing respiratory illness. Like with cardiovascular issues, there seems to be a strong link between PM10 exposure, inflammation, and respiratory illnesses. Several studies have linked exposure to PM with an increase in inflammation as an immune response. In some cases, excessive inflammation leads to an increase in reactive oxygen species, nitrogen species, and release of cytokines. Taken as a whole, chronic inflammation due to PM exposure has been shown to lead to morphological changes in respiratory pathways, asthma, and chronic obstructive pulmonary disorder.37
Because PM2.5 can penetrate more deeply into the respiratory system, it has been linked with the development of lung cancer. PM2.5 also tends to have greater proportions of mutagenic species, making cancer a more likely outcome of exposure than the mostly biological and mineral composition of PM10. In fact, cancers of the respiratory system (including the trachea, bronchus, and lungs) accounted for seven percent of total mortality due to PM2.5 exposure in 2010. More research is needed on the explicit links between PM and various types of cancer.38
Coal workers’ pneumoconiosis, more commonly known as black lung disease, is a unique impact of PM. As the name suggests, it is common in coal miners and others who work in the coal industry. It occurs as coal dust builds up in the lungs, leading to inflammation, formation of scar tissue, and death of lung cells. Black lung disease is a progression and often goes unnoticed or undiagnosed since symptoms mimic more common respiratory illnesses. Unfortunately, it is uncurable and in its most advanced stage is often fatal. And though the number of deaths attributed to black lung disease has fallen since 1990, recent studies have shown a resurgence in cases.39
Acid Mine Drainage
Acid mine drainage (AMD) forms when sulfide minerals are exposed during mining or other large-scale excavations. These minerals oxidize when exposed to air and water and are converted into sulfuric acid, metal ions, and sulfate. These new chemical species can then enter surface water or groundwater where they can have serious ecological impacts.40 The chemical makeup of AMD varies, but it generally has a pH ranging from 2 to 8, and consists of group II metals, transition metals (especially iron and aluminum), and either bicarbonates or sulfates.
AMD forms in four steps: 1) oxidation of pyrite by atmospheric oxygen, making sulfate and ferrous iron, 2) conversion of ferrous iron to ferric iron, 3) hydrolysis of iron leading to the formation of iron hydroxide, and 4) additional oxidation of pyrite by ferric iron. Because oxygen is not the oxidizer in the fourth step, this process can be self-perpetuating so long as sufficient pyrite is available. The second step in the process is considered rate-limiting and controlled by the presence of specific bacteria.41 Other sulfur-bearing minerals can undergo similar oxidative reactions leading to the formation of AMD, including pyrrhotite, chalcopyrite, arsenopyrite, sphalerite, and galena.42 AMD is a worldwide concern because of the sheer number and distribution of coal mines. Studies have shown AMD-related damage in Asia, New Zealand, Europe, South America, Canada, and the United States. In the eastern U.S. alone, 10,000 km of streams/rivers and 77 hectares of lakes/reservoirs have been damaged by AMD.43 Specifically, AMD is problematic where abandoned mines are left without reclamation and are exposed to air and water or where mine waste breaches containment. This is more common in countries with weak environmental regulations or enforcement of those regulations. Recently AMD has devastated water bodies in South Africa where coal accounts for over 90% of the country’s electricity generation.44,45
Environmental Impacts
AMD has several different environmental impacts. Constituents of AMD can be directly toxic to aquatic and terrestrial organisms. AMD can alter the biogeochemical nature of habitats, stain stream sediments, disrupt nutrient cycles, and render water unfit for domestic, agricultural, or industrial use.46 The low pH of most AMD has several ecological consequences. Terrestrial plant species are impacted as nutrient availability changes in response to the presence of acids. Aquatic organisms typically experience sub-lethal toxicity as the pH decreases, but below a certain threshold pH (around 5 for many sensitive organisms), organisms begin to die off or migrate elsewhere if possible. Perhaps more importantly, acids tend to mobilize heavy metals. In plant life, exposure to or uptake of heavy metals causes oxidative stress that can lead to cell damage and morphological changes. At high enough levels, metals can cause plant death. In aquatic organisms, many metal species can cause immediate toxicity. Such metals include cadmium, copper, lead, and zinc. Other metals species stunt growth, reduce reproductive success, and lead to deformities in offspring. In humans, heavy metals are known to disrupt metabolic functions, accumulate in vital organs, and block absorption of other minerals.47
Acid Deposition
There are four main processes in acid deposition (Figure 5). First, SO2 and NOx are released into the air from combustion of fossil fuels. The combustion of coal in coal-fired power plants is a significant source of SO2. Once in the atmosphere, this material reacts to form acid particles that can travel long distance via atmospheric circulation patterns. Eventually they fall to the earth through wet and dry deposition. Wet deposition occurs when the particles fall during rain and/or snow events, while dry deposition occurs as dust particles settled out of the atmosphere. Once into terrestrial or aquatic ecosystems, the acid can have harmful effects.

Figure 5: Processes of acid deposition: 1) emissions of SO2 and NOx are released into the air, where 2) they are transformed into acid particles that may be transported long distances. 3) These acid particles then fall to the earth as wet and dry deposition (dust, rain, snow, etc.) and 4) may cause harmful effects on soil, forests, streams, and lakes.48
Coal combustion is not the only source of the raw materials that lead to acid deposition. Other anthropogenic activities include the combustion of gasoline in automobiles and combustion of other fossil fuels such as oil or natural gas to generate electricity.49 There are also natural sources that emit these compounds, most notably volcanic activity.
Environmental Impacts
Acid deposition is problematic for some of the same reasons as AMD. The introduction of acids can lower the pH of aquatic and terrestrial environments. For example, as acid rain flows through soil it can leach out aluminum from soil particles and transport it to streams and lakes where it is toxic to aquatic organisms. As discussed earlier, the threshold pH for most aquatic organisms is around 5. Below this, many organisms suffer increasing toxic effects. Acid rain also leaches out nutrients critical for terrestrial plants. In the absence of these nutrients, plant life is greatly diminished, which negatively impacts food chains and can lead to enhanced soil degradation. Less-vegetated soil dries out faster, is less stable, and is more likely to erode since there is less of a root network to hold soils in place during rain or wind events.50 Many of the most pH-sensitive organisms are critical to ecosystem function and their absence leads to significant ecological decline. Acid rain is especially problematic for environments lacking in buffering capacity – the ability to resist changes in pH. This was evidenced by the decline in sensitive macroinvertebrate, fish and aquatic plant species in Quebec, Canada in the 1980s and 1990s partly as a result of coal combustion in the Midwest of the U.S.51 This region was particularly vulnerable to the impacts of acid deposition because its lakes and streams are naturally low in dissolved minerals that serve as buffers. Emission standards set by the U.S. and Canada have decreased sulfate concentrations and led to a rebound in aquatic ecosystems.52
Mercury Emissions
Small amounts of mercury are contained in the coal burnt in electricity-generating power plants. This mercury settled out of the atmosphere into the sediment or was accumulated into the original plant tissue hundreds of millions of years ago during the Carboniferous. When the resultant coal is burned in coal-fired power plants to generate electricity that mercury is returned to the atmosphere. This is not problematic on its own. But when the mercury settles into water or onto land, several classes of microorganisms change it into methylmercury, a highly toxic and persistent form of the element. Mercury from coal-fired power plants accounts for 44% of all mercury emissions in the U.S. (EPA), the most of any source. Non-coal sources of mercury include but are not limited to gold mining, waste incineration, and chlorine production. Collectively their emissions levels are much smaller and less significant than those from burning coal.53
Environmental Impacts
Because methylmercury is highly bioavailable and persistent in the environment, it can be bioaccumulated and biomagnified. Bioaccumulation occurs when an organism takes up a toxin that accumulates in its tissue because it cannot be digested or processed by the body. Bioaccumulated material is typically stored in the fatty tissues of organisms. Biomagnification occurs when the concentration of a bioaccumulated toxin increases up the food chain. This occurs because higher trophic level organisms eat many of the lower trophic organisms. This has the effect of concentrating the toxin at the higher trophic levels and leading to greater toxicity.54 Because much of the mercury emitted into the atmosphere settles into the ocean, aquatic species tend to accumulate greater concentrations of mercury. This is especially true of shellfish and cold-water fish due to their fatty nature. Anything that eats these organisms is at risk for mercury toxicity, including humans.55
In the body, mercury acts as a neurotoxin and can lead to a loss of peripheral vision, pins and needles feelings in extremities, loss of motor function, and muscle weakness. Because it is a neurotoxin it has particular adverse effects on infants and children. Exposure to mercury at developmental stages can lead to long term effects on cognitive thinking, memory, attention, language, fine motor skills, and visual spatial skills.56

Comments: