Two of the most difficult topics for students are photosynthesis and respiration, both of which have an effect on the carbon cycle and global warming. Respiration is the breakdown of organic compounds, which provides energy for the cell and releases carbon dioxide back into the atmosphere. The process of photosynthesis removes carbon dioxide from the atmosphere, sequestering it in organic compounds. It also converts light energy into chemical energy, making the Sun's energy available for all living things on Earth. Did someone say energy? Don't we have an energy crisis now? Aren't we paying over $3.00 a gallon for gas to run our cars? Can't we do the same thing that plants do with solar energy, or is that different? And hasn't that guy, Al Gore, been saying something about global warming and how we need to get excess carbon dioxide out of the atmosphere before the sky falls? Do plants and other photosynthetic organisms know something that we don't know?
These are some of the questions I want to try to have my students think about and discuss as we look at just how plants do photosynthesis. I plan to introduce them to current ideas about how to recreate this natural process through artificial photosynthesis—an idea that may help to solve some of our energy and global warming issues. But to understand how artificial photosynthesis might work, they have to understand the basics. So we are back to, "How do I find a way to teach photosynthesis in a way that students can understand?" I have used a variety of methods—diagrams, videos, and labs. Diagrams generally involve chemical symbols, arrows, and cycles drawn on two-dimensional paper, which loses all connection with the plants that these reactions are happening in. Videos have left my students and me similarly less than satisfied. One video I have used even reduced the Calvin Cycle to a bunch of gears. Yes, it was an analogy, but not one that most students could quite understand. Typical experiments involving photosynthesis show the results of what is happening, but again, it turns the cell into a little black box where carbon dioxide comes in and glucose and oxygen go out. The most success I've had was when I used props and my students to act out the processes involved in photosynthesis, turning the classroom into a chloroplast. With this method, I finally achieved a few "aha" moments. I believe it was the three-dimensional aspect of that desperate attempt to enlighten my students that helped them visualize what diagrams and videos and reactions in test tubes did not. In this unit, I will refine those models and props to create a three-dimensional approach to teaching photosynthesis. Once they can visualize the invisible, the models will then be used to tie in the actual processes to what students are observing in lab, and will provide a better basis for the formation of hypotheses and conclusions in inquiry labs. Based on the model and role-playing, students could then develop their own simple models for demonstrating the variations in photosynthesis by C4 and CAM plants.
Following this unit, we will be discussing global warming, and how deforestation and our dependence on fossil fuels are contributing to the problem. Normally, I would teach environmental issues such as global warming along with a unit on ecology, either at the beginning of the course, or at the end. By coupling this topic with photosynthesis, I believe it will help students to make better connections between the causes, effects and possible solutions to this problem. How does cutting down trees have anything to do with global warming? Where does the energy come from that is in fossil fuels?
I teach in an urban high school with a population that is about 46% African American, 39% White, 11% Hispanic and 4% other. I am designing this unit for my AP and IB classes, but plan to use the model with a less detailed explanation of the process, with my Biology I classes. The upper-level biology courses are very diverse, reflecting the school's population. The objectives to be addressed for the AP Biology curriculum include 2.03—an examination of subcellular organelles and how their structure relates to their function, and 2.02—analyzing cellular membranes and mechanisms of transport. The model used in this unit will be a reflection of our current understanding of the structural components of a chloroplast. Objective 3.01 requires that students analyze and examine the purpose, and adaptations of bioenergetic reactions such as photosynthesis, the main focus of this unit. In addition, this unit will cover several AP objectives for the scientific process. Objective 1.01—identification of questions and problems that can be answered through scientific investigations, and objective 1.02—designing and conducting scientific investigations, will be covered as students use what they have learned through models and role-playing to make and test hypotheses. Finally, this unit will address objectives related to ecological principles. Objective 7.02 requires students to examine interactions in ecosystems, including energy flow and element cycling, and objective 7.03 has students assess global issues such as global warming and how human populations can affect the environment.
The goals and objectives for the IB Biology HL are similar to the AP objectives. This unit will reinforce what the IB students will have already learned about membrane structure, the types of membrane proteins, and transport mechanisms across a membrane in Goal 1.4. Goals 2.8 and 7.2 in the IB curriculum address the nature of light energy, photolysis, the light and dark reactions, and the adaptations of C4 and CAM plants. As part of the IB Biology curriculum, I select two topics for more in-depth study. One of these will be Ecology, which includes objectives on energy flow in ecosystems and the carbon cycle (Goal 4.1 and G2), the effects of human actions on the greenhouse effect and what we can do to reduce global warming (Goal 4.5), and how biomass can used as a source of fuel (Goal G5). Here, I hope students will be able to make connections between what they have learned about photosynthesis and how it might be applied to solving the global warming problem, as well as creating renewable energy resources.
Background Information
The main focus of my research has been on photosynthesis and what is currently understood about this process. I think that even teachers often have a difficult time with this topic because we ourselves do not fully understand it. During my time working on this unit, I have tried to clarify my understanding and correct the misconceptions I have had about what happens at the molecular level during photosynthesis. There have been many surprises and many "aha" moments for me, as I have found answers to how some of the steps of photosynthesis work. There is much research still going on about specific molecules and the role they play. So, at some level there are still some black boxes, but they are much smaller and being illuminated all the time by the research being done today.
The following is a summary of what is currently known about photosynthesis—at least to the level at which I want to take it. As I go through this information, I will refer to diagrams of the model and how it and the other activities can be used to demonstrate to students how things are happening. Much of this background information is very detailed. I would not necessarily provide all this detail to my students, or expect them to know all the names of the molecules involved. But, as every teacher knows, you always have one or two students each year who really want to go deeper and understand why something happens the way it does. Now, I feel I have a little better information to help me answer their questions.
Chloroplast Structure
Most of photosynthesis happens in the chloroplasts. The chloroplast is surrounded by an outer double membrane, inside of which are the thylakoids, where the light-dependent reactions occur. (My model does not show this outer membrane, but only the thylakoids. See Figure 1. The outer layer will be represented by covering the model with a double layer of fabric, which is removed after discussion of that structure.) Surrounding the thylakoids is the stroma—an enzyme-filled fluid where the light independent reactions take place. I had always taught that the thylakoids were stacks of individual sacs like pita pockets. This is not true at all. The thylakoids are made from a long continuous membrane folded back on itself in such a way that pockets are created between the layers of membrane. Compressed stacks of these pockets are called grana and are connected by unfolded layers of thylakoid membrane—the lamellae. (Here I plan to use a long piece of fabric, with "right" and "wrong" sides, folded back and forth to create a granum. See the "Fabric Thylakoid Demonstration" in the Lesson Plan section.) There are two different
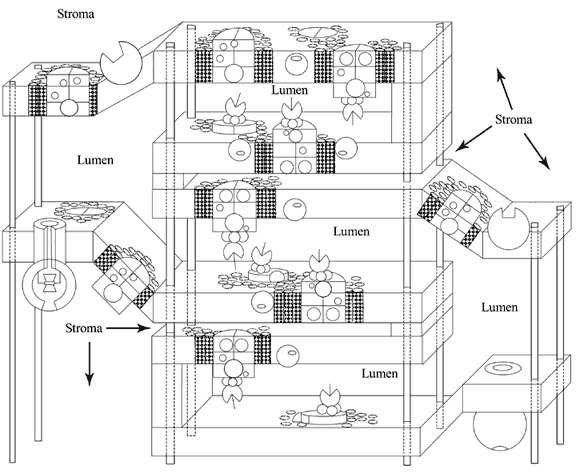
Figure 1 — Model of thylakoid membrane
sides to this membrane—one that faces the stroma and one that faces the space created by the folding of the thylakoid membrane, called the lumen [1, 2]. (After demonstrating the two sides of the thylakoid with the fabric demonstration, I will have students find the stromal and lumen sides of the membrane on the model.) In the thylakoid membranes are clusters of photosynthetic pigments—Photosystem I (PS I) and Photosystem II (PS II), along with proteins, which create an electron transport chain, and ATP synthase. (See Figure 2 for details of these components.)
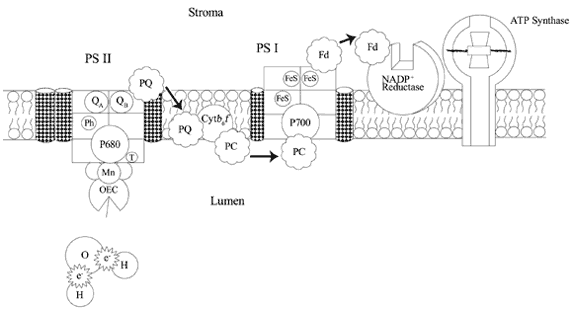
Figure 2 — Thylakoid details
These photosystems contain light absorbing pigments such as chlorophyll and carotenoids, associated with proteins, which are involved in harvesting and converting light energy into chemical energy. At the center of both photosystems are special chlorophyll a and protein complexes known as reaction centers because they respond to the energy of light, either directly or by transfer of energy from antenna pigments, by donating electrons that have absorbed the light energy and been boosted to excited energy levels. (The reaction centers are represented in the model by the Styrofoam cylinders. The hair curlers represent the antenna pigments.) The reaction center of PS I contains a chlorophyll a molecule whose maximum absorption occurs with light at a wavelength of 700 nm. The chlorophyll a molecule in the reaction center of PS II has a maximum absorption wavelength of 680 nm. Both chlorophyll molecules are associated with dimer (two-part) protein complexes. (The two halves of the cylinders represent these dimers.) PS II is most often found in the center of the grana and PS I is found near the ends of the grana or in the connecting membranes of the lamellae. This seems to be related to the structure of the two photosystems. You can see from diagram of the model in Figure 1 and the details of PS I and PS II in Figure 2, that the two photosystems do not sit in the membrane in the same way. PS I extends further out into the stromal side of the membrane and PS II extends out into the lumenal side of the membrane. The grana membrane has mostly PS II complexes, which allow the membrane to stack closely together on the stromal side of the membrane. (Notice the much narrower space between the stromal-side membranes in the grana section of the model in Figure 1.) This would not be possible with the PS I complexes. There also is some evidence that the antenna pigments of PS II complexes connect where the membranes fold back in the grana. This would allow light energy to be transferred to reaction centers across membranes as well as laterally. Because of their structure, PS I and ATP synthase are relegated to the lamellar membranes and to the ends and outer membranes of the grana [2]. Around the reactions centers are clusters of chlorophyll a and b and carotenoids associated with proteins in Light Harvesting Complexes (LHC's) that form a solar-collecting antennae that absorbs and transfers light energy to the reaction centers of the two photosystems. Why are antenna pigments needed? Why doesn't every chlorophyll molecule give off electrons and participate in the light reactions? Well, a photon is absorbed by a chlorophyll pigment about every tenth of a second on a good day—which is not very frequent. Each chlorophyll would also require all the other components necessary for the conversion of light energy to chemical energy—components that would be costly compared to the amount of energy they would be capturing. But, if the energy absorbed by a lot of pigments can be passed to one reaction center, then that one pigment can be kept busy [3]. Antenna pigments may surround one reaction center, or be associated with more than one, so that energy may be passed from one antenna pigment to one of several reaction centers. It also appears that at least one of the LHC of the antenna complexes moves from PS II to PS I and back again to aid in making the most efficient use of the light. If PS II is getting so much light absorbed by its antennae pigments that it cannot replace its electrons quickly enough, then LHCII will shift over to the part of the thylakoid membrane where PS I is located and temporarily dock with it, reducing the amount of light collected for PSII and so that more light energy is being collected and passed to PS I [2].
Photosynthesis occurs in two main phases. The light dependent reactions involve the photosystems and proteins of the thylakoid membrane, and convert light energy into chemical energy. The following equation summarizes the oxidation of water, which happens in the light reactions:
2 H 2O + light energy g 4 H + + 4 e - + O 2
The oxidation of water provides the supply of electrons that keeps the photosystems running. It is also the source of most of the oxygen in the atmosphere.
The light independent reactions occur in the stroma of the chloroplast and involve the enzymatic fixation of carbon dioxide and synthesis of a three-carbon sugar using the energy captured during the light reactions. This does not require light, but there may be light dependent regulation of this process [3]. The equation for the reduction of carbon dioxide is:
CO 2 + 4 H + + 4e - g (CH 2O) n + H 2O
The overall reaction for photosynthesis is shown below:
6 CO 2 + 6 H 2O g C 6H 1 2O 6 + 6 O 2
However, even though glucose is shown as being an end product, the final product in most plants is triose phosphate (a three carbon sugar), sucrose, or starch. The point is that an organic compound is made. Again, as I go through the steps, I will refer to the model and how it can be used during lecture.
The Light Dependent Reactions—Noncyclic Electron Transport
An antenna pigment in Photosystem II absorbs a photon of light energy. If the pigment is near the outer edges of the cluster of pigments, it is probably a high-energy blue wavelength absorbing pigment. Pigments closer in to the reaction center will absorb lower-energy red wavelengths of light. This creates a gradient of energy absorption. As energy is transferred through these antenna pigments, it passes from higher energy-absorbing pigments to lower energy-absorbing pigments, losing just a little heat energy with each transfer. In this way, they "funnel" the absorbed energy to the reaction center. The energy is NOT passed in the form of high-energy electrons, but physically, such as the way one vibrating tuning fork can cause another to vibrate, or the way one pool ball striking another, stops, but causes the second ball to move. Electrons do not get involved until the energy reaches the reaction center [3]. (To get things started, I will shine a light from a lamp onto the model. Mounting the "curler" antenna pigments onto the Styrofoam with little springs, will allow me to wiggle them to show how energy might be passed from one to the other, and then to the reaction center.)
When the energy from a photon of light is received by P680 in PS II, an electron is boosted to a higher energy level—an excited state. (The model must be set up with one of the fuzzy electrons attached to PS II.) The excited state only lasts for a billionth of a second, so it is necessary for the electron to pass from P680 to an acceptor molecule before it falls back to a lower energy level, releasing its energy as heat. In this excited state, P680 becomes much more reductive and reduces the acceptor molecule, a nearby chlorophyll-like component of the reaction center—pheophytin—by transfer of the excited electron. (Transfer the electron from P680 to the pheophytin golf tee.) This makes the pheophytin negatively charged and P680 positively charged. At this point the electron must be transferred very quickly away from pheophytin or the electron will transfer back to P680 +. This is accomplished by the fast transfer of the electron from pheophytin to a quinone molecule on the stromal side of the reaction center—Q A—thus separating the charged molecules by putting some distance between them. (Transfer the electron from pheophytin to the purple Q A ball on PS II.) The electron is then passed to a second quinone—Q B. (Transfer the electron to the yellow-orange Q B ball.) A lipid soluble mobile electron carrier, plastoquinone (PQ), docks in the Q B site until it has picked up two electrons, along with two hydrogen ions from the stroma. (PQ is represented by a large black pompom with two small patches of Velcro glued on to pick up the electrons and hydrogen ions. I have used large pompoms for molecules that are moving in this model. Now if PQ has to pick up two electrons, this means that two photons of light have to be absorbed. But at this point, P680 + has no more electrons to give off. That will be fixed shortly, but in the meantime, I will have one electron already on PQ. Notice that for PQ to pick up electrons in the Q B site, it will be located on the stromal side of PS II, where it will also pick up the two hydrogens—small Styrofoam balls with Velcro.) PQ, now loaded—reduced—with two electrons and two hydrogen ions, detaches from the Q B site and PSII and diffuses within the lipid layer to the luminal side of the membrane. There, when it encounters a molecule of cytochrome b 6/f in the membrane, it transfers its two electrons and the hydrogen ions are released into the lumenal space. One of the things that surprised me was how several different types of molecules in the thylakoid membrane actually move around in order to transfer electrons. (Move PQ down through the membrane to cytochrome b 6/f on the lumenal side of the membrane. Pass the electrons to the cytochrome and release the hydrogens into the luminal space.) As this process continues, hydrogen ions accumulate in the thylakoid lumen, which will later be used to synthesize adenosine triphosphate (ATP). Here was another misconception straightened out—I thought there was a proton pump that was using the energy of the electrons to pump protons across the membrane. Plastoquinone is more of a carrier than a pump. Another mobile electron carrier molecule, located in the luminal space is plastocyanin (PC). PC picks up electrons, one at a time, from cytochrome b 6/f and transfers them to the reaction center P700 + of Photosystem I, making the trip up to a thousand times per second [6]. P700 + would be positively charged because I would be starting PS I from the point where it had already lost an electron from previous excitation by light. (Transfer one of the low-energy electrons from the cytochrome to PC—represented by a large blue pompom with attachment sites—and then to the reaction center P700 +.) By this time the electrons have lost a lot of the energy they had gained from the light as they have been passed from molecule to molecule. A photon of light is absorbed by the antenna pigments of PS I, and the energy is passed from one to the next until it is absorbed by the reaction center chlorophyll, P700, which excites an electron. (Shine the light onto PS I, wiggling the "curler" antenna pigments.) The electron is then passed within the reaction center protein complex through several subunits—chlorophyll Ao, the primary acceptor, quinone A 1, (not included in this model) and then to several iron and sulfur containing proteins [3, 7]. (Remove the electron from P700 and pass it along the Fe-S proteins represented by the hot pink golf tees.) At this point the electron is picked up by another electron carrier, located in the stroma—a ferrodoxin protein (Fd)—which transfers two electrons, one at a time, to ferrodoxin-NADP + reductase (FNR) on the stromal surface of the thylakoid membrane. (One of the electrons should already be placed on FNR, ready for the second to arrive. FNR is represented by a white Styrofoam disk with a notch cut out for NADP +. Ferrodoxin, as a mobile electron carrier, is represented by a large red pompom with an attachment site for one electron. Transfer the electron from the last Fe-S golf tee protein to the red pompom, Fd, and then place Fd on its binding site on FNR.) FNR reduces NADP +, from the stroma, to NADPH with the addition of the two electrons and one hydrogen ion from the stroma.
2 Fd r e d + NADP + + H + g 2Fd o x + NADPH
NADPH, along with ATP, is used in the light-independent reactions to reduce CO 2 to sugar [3]. Since these reactions take place in the stroma of the chloroplast, it also makes sense that this reaction takes place on the stromal side of the thylakoid membrane. Structure is related to function! (Attach the K'nex connector, representing NADP +, to the notch in FNR, and attach a hydrogen ball from the stroma and the two electrons onto the NADP + molecule, creating NADPH. Release the Fd pompom from FNR.)
At this point, both reaction center molecules have given off electrons that have to be replaced before the process can begin again. The P680 reaction center became positively charged once the excited electrons were transferred to the pheophytin and were sent on their way to PS I. Adjacent to P680 + is a tyrosine amino acid (Tyr z) which donates an electron to P680 +, returning P680 to its neutral state, but Tyr z also gives off a hydrogen ion, making Tyr z very reactive. (Tyrosine, represented by a brass thumbtack, should have an electron on it as the demo begins. Remove this electron and transfer it to P680.) Tyrosine then accepts a proton and an electron from a structure containing 4 manganese atoms on the luminal side of PS II, called the oxygen-evolving complex (OEC). Two water molecules bind to the OEC—perhaps to the four, now positively charged, manganese ions—and are oxidized to form O 2. The electrons from water replace those lost by the manganese ions, and the hydrogen ions are released into the thylakoid lumen where they contribute to the hydrogen ion concentration [1]. (Take a water molecule and attach it to the OEC by placing the oxygen onto the toothpick. Pull off the hydrogen ions and release them into the lumen. Place the two fuzzy electrons onto two of the pale yellow manganese balls of the OEC. Release the oxygen ball into the lumen. Do the same for one more water molecule. Take the two oxygen atoms and put them together with a toothpick and release this oxygen molecule from the chloroplast. Remove an electron from one of the manganese molecules and pass it to Tyr z.) Light does not directly activate the OEC to split water. It is the light captured by PS II and the subsequent oxidation of the reaction center that starts the sequence of redox reactions that lead to water being split as its electrons are removed [8]. Electrons, from the oxidation of water, therefore, pass through both PS II and PS I in a linear fashion, until they are ultimately accepted by NADP + to form NADPH.
ATP is made using the energy of the proton gradient established by plastoquinone's transferal of hydrogen ions from the stroma to the lumen of the thylakoid during electron transport from PS II to PS I. Hydrogen ions are also released into the lumen when water is split to release oxygen, and electrons for P680 +. This accumulation of hydrogen ions creates both a difference in charge (electrical potential) and a difference in pH (chemical potential) across the thylakoid membrane. Together these are called the proton motive force [3]. This force provides the energy used by the ATP synthase enzymes, located in the stromal lamellae membranes, to phosphorylate ADP to ATP as the hydrogen ions diffuse back across the membrane through the enzyme. Since the initial energy necessary to transport the electrons and protons came from light, this form of ATP synthesis is called photophosphorylation. Although there is still much to learn about how ATP synthase uses the flow of hydrogen ions to make ATP, it is known that there is a part of the enzyme that rotates as the protons go through. This rotation may cause a conformational change in other parts of the enzyme with the result that ATP is produced from ADP and inorganic phosphate. The rate of ATP production is about one ATP produced for every three to four hydrogen ions that are moved through the enzyme [3]. (ATP synthase is represented by the noodle/paddle-wheel filled Styrofoam ball combination. The paddle wheel represents the rotating part of the enzyme; however, it is probably oriented in the wrong direction, and should be in the portion of the enzyme that is embedded in the membrane. For purposes of demonstrating ATP synthesis, I chose to put it in this direction so I could drop hydrogen ion balls through, turning the paddle wheel, much as water turns a water wheel. It would be very difficult to show ATP being made with this model, so at this point I will break away from the model and just tell the students that we don't quite know how ATP gets made, but show them my favorite representation of ATP—the ATP gun. (See the Appendix for a description of this demo.)
Cyclic Transport
Sometimes, electrons do not flow from PSI to NADP +, but travel in a circular path so that they end up back on PSI. In this case, the excited electrons from P700 are transferred through the photosystem, but instead of being transferred to ferrodoxin reductase, they are picked up by plastoquinone and travel through the pathway that returns the electrons to P700 + at the energy level they were at before excitation. But just as in the noncyclic pathway, hydrogen ions are transported by PQ across the thylakoid membrane, setting up the conditions for chemiosmosis and ATP synthesis. No oxygen is produced, nor is NADP + reduced in this cyclic pathway [4]. This pathway will provide the cell with energy in the form of ATP, but without the hydrogens provided by NADPH during the Calvin cycle; its purpose may be to simply make up for a shortfall of ATP provided by noncyclic electron transport so that there will be enough for the synthesis of carbohydrates in the Calvin cycle [5]. (To demonstrate cyclic flow, pass an electron from PS I along the Fe-S golf tees proteins, to the red ferrodoxin, and then to the black PQ. After picking up another electron, PQ must pick up two hydrogen ions from the stroma, and then release them into the lumen as the electrons are transferred to cytochrome b 6/f. The electrons are then picked up by the blue PC pompom and transferred back to P700 +, completing the cycle. The hydrogen ions can be used to make ATP when they diffuse out through ATP synthase.)
The Calvin Cycle or Light-Independent Reactions
The second stage of photosynthesis takes place in the stroma of the chloroplast and involves the fixation of carbon dioxide and the production of sugars using the high energy molecules ATP and NADPH produced in the light reactions. The Calvin cycle can be divided into three stages: carboxylation (carbon fixation), reduction, and regeneration [3]. (Here, I leave the model, except to tell the students that we are taking NADPH and ATP out of the thylakoids and bringing them further out into the stroma—which is now the classroom area around the thylakoid model. The idea of a cycle is often taken too literally by students. To help them understand that the steps of the Calvin cycle are all occurring simultaneously, I will have them simulate a cycle with the activity "Kool-Aid Cycle". (See Lesson Plans, Day 5) Once they realize how cycles work, I will go through the steps of the Calvin cycle as students play the roles of the molecules involved as described in the Lesson Plan section, Day 5.
In carboxylation, an enzyme called rubisco (ribulose-biphosphate carboxylase/oxygenase) is required in order to fix carbon dioxide gas coming into the chloroplast. It is probably the most abundant protein on the planet. Chloroplasts are full of it and there are a lot of plants. They need a lot because it is not a very efficient enzyme. It can fix only a few CO 2 molecules per second. In addition, O 2 can also combine with rubisco in place of the CO 2, reducing the plant's photosynthetic ability. It may be that rubisco evolved in anaerobic bacteria when there was little oxygen in Earth's atmosphere. As more oxygen was released into the atmosphere, the enzyme lost much of its efficiency due to competition with O 2, but not enough so that it was selected against [3].
A molecule of carbon dioxide gas is combined with a five-carbon compound called ribulose 1,5-biphosphate, or RuBP by the enzyme rubisco. This forms a very unstable six-carbon compound which immediately breaks down into two, three-carbon compounds—3-phosphoglycerate (PGA). This must occur with two more CO 2 molecules in order for the final product to be made—a triose phosphate sugar.
In the reduction stage, a series of enzymatically-catalyzed reactions uses the products of the light-dependent reactions to reduce PGA. The 6 (3C)-PGA molecules are first phosphorylated using six ATPs to produce glycerate 1, 3-bisphosphate (GBP). Each GBP is then reduced when NADPH is used to replace one of the phosphate groups with a hydrogen atom. Six (3C) glyceraldehyde 3-phosphates (G3P) are formed. Five of these, phosphorylated by three ATPs, are enzymatically shuffled around in the regeneration stage to reform the 3 (5C)-RuBP molecules. One will be retained as a triose phosphate and be used in forming either a starch molecule in the chloroplast, or a sucrose molecule in the cytoplasm. They leave the chloroplast through translocator proteins in the chloroplast membrane that exchange triose phosphate molecules for inorganic phosphates from the cytoplasm [3].
Photorespiration, C4, and CAM Plants
As I mentioned before, the enzyme rubisco, fixes CO 2, but will also bind with O 2, producing one PGA and a phosphoglycolate. The reactions that eventually convert phosphoglycolate into PGA are costly in terms of energy and result in one less CO 2 molecule being fixed. This process is called photorespiration. It often occurs when temperatures are high and conditions are dry. To reduce evaporative water loss, plants will close their stomata, which keeps the water in, but also keeps in the O 2 produced in photosynthesis, and keeps out more CO 2. Oxygen levels build up in the leaves, making it more likely that rubisco will bind with O 2 rather than CO 2 [3].
Plants that carry out the Calvin cycle and produce a 3C PGA when they combine CO 2 with RuBP are known as C3 plants. These kinds of plants are most affected by photorespiration, and include many crop plants like wheat and potatoes, and plants of temperate climates. Other types of plants have evolved ways of dealing with this problem. C4 plants (sugar cane, corn, and other grasses) have special cells near the vascular bundles or veins, called bundle sheath cells. These plants fix CO 2 in the mesophyll cells by combining CO 2 with a 3C compound, phosphoenolpyruvate (PEP) to form a 4C oxaloacetate instead of PGA, hence the name C4 plants. Oxaloacetate is then reduced using NADPH to form malate, which is transported to the bundle sheath cells. In the bundle sheath cells, the malate is decarboxylated (meaning the CO 2 is released) into pyruvate and CO 2, and the NADPH is regenerated. The pyruvate is transported back to the mesophyll cells where it is converted back into PEP. Rubisco is found in the bundle sheath cells and takes advantage of the higher concentrations of CO 2 present to carry out the Calvin cycle. There is a cost to the plant because of the energy required in the transport of molecules from mesophyll to bundle sheath cells. This cost is offset by the advantages of having to deal with less photorespiration when conditions are hot and dry. Then the C4 plants can afford to close up their stomata, reducing water loss, because they have secured high concentrations of CO 2 in the bundle sheath cells [3].
CAM (crassulacean acid metabolism) plants reduce water loss and avoid photorespiration by keeping their stomata closed during the day and open them at night to let in CO 2. During the night the CO 2 is fixed in the mesophyll cells by combining with PEP. It then goes through a series of enzymatic reactions, which convert it into malate. The malate is stored in the vacuole of the cell as malic acid. In the daytime, the malic acid is released from the vacuole and is transported into the chloroplast where CO 2 and PEP are released by decarboxylation, and the Calvin cycle is carried out, while stomata are closed. However, there is an energy cost to making malate, and the vacuole can only store so much malic acid. The plant may not get as much CO 2 as if it had kept its stomata open, so growth tends to be slow. CAM plants include cacti, pineapples, and other succulent plants [3].
One benefit to the higher CO 2 levels that are creating global warming is that C3 plants will be better able to avoid photorespiration because of the improved CO 2 to O 2 ratio. Higher temperatures, however, may increase evaporative water loss and offset the advantages.

Comments: