Lesson Plans
Day 1—Leaf Structure
Warm-up Questions
What might be some ways that plant cells would look different from animal cells? What are some reasons we need plants? If plants could talk, what might they say about needing us? What might plants need to live? How might they get these things?
Students start the unit by learning about leaf structure. First, they are required to make a diagram of a leaf cross-section and a chloroplast using their textbook and labeling and writing descriptions of the functions of all the parts. This can be done as a homework assignment the previous night to give more class time for the lab work. Once they are familiar with what they should be looking for, I have them observe, draw and label prepared cross-sections of leaves using a microscope. After a review of the function of stomata from their diagram assignment, students then will observe stomata under the microscope. The best plant for this is called Wandering Jew, Tradescantia zebrine. It has leaves with a lot of very bright purple pigment on the underside, but the guard cells show up as bright green. There is no mistaking them. Students do not even have to peel the leaf. All that is required is to make a wet mount slide of a small piece of the leaf with the underside facing up. Students draw, measure, and count the number of stomata per unit area of leaf. They also observe what happens to the stomata when a solution of salt water is drawn under the cover slip, causing the guard cells to lose turgor pressure and close the stomata. Once they know what to look for, I ask students to predict how the stomata might be different on different types of leaves. Will size vary? Number? Location? Shape? They then select several different types of leaves and test their hypotheses by recording their observations and conclusions in their lab notebooks. Class closes with a discussion of the results.
The night's assignment includes having students read background information on the making of starch pictures—one of the next day's labs. This information can be found at the following website:
http://www.oxygraphics.co.uk/starchpics.htm
Day 2—Chloroplast Structure
Warm-up Questions
What kinds of plant parts would you expect to find chloroplasts in? What might someone say that pigments are? Where might you find pigments in plants? What pigments would you expect to find in leaves? What seems to be happening when leaves change color in the fall?
Cytoplasmic Streaming
I want students to observe real chloroplasts. The easiest plant to do this with is the American waterweed, Elodea canadensis, sometimes sold as Anacharis, Egeria densa. I often have students observe this plant under the microscope, but sometimes they are unable to find the chloroplasts involved in cytoplasmic streaming. In case they have trouble, I use a short video clip of cytoplasmic streaming of chloroplasts. The following website has a very good video loop that clearly shows the chloroplasts:
http://www.microscopy-uk.org.uk/mag/artnov00/dwelodea.html
I have students measure the chloroplasts using the microscope and make drawings of them. They determine an average number of chloroplasts per cell, and describe which cells they see cytoplasmic streaming in and how the chloroplasts are moving in those cells. They are then asked to hypothesize about the following questions: Why some cells in the same leaf have cytoplasmic streaming and others do not. Why is cytoplasmic streaming observed in the Elodea and not in the Wandering Jew plant observed on the previous day? What could they do to increase the speed of cytoplasmic streaming in the Elodea cells? Observations and responses are recorded in their lab notebooks.
Starch Pictures
In order to show the distribution of chloroplasts in a leaf, and to demonstrate that starch is stored in the chloroplasts following photosynthesis, I will have students create starch pictures on leaves. This is done by exposing the leaves of a geranium plant to a negative or slide projected from a slide projector after the plant has been stored in the dark for 48 hours—depleting its starch reserves. The plant will begin to make starch again, but only in the chloroplasts receiving the light coming through the light areas of the negative. Iodine staining reveals the areas of the leaf where starch has been produced, and creates the image. Detailed instructions can be found at the following website:
http://www.accessexcellence.org/AE/AEC/AEF/1996/morishita_pictures.html
I will have students set up the leaves in the glass plates with the image projected on them in our darkened stockroom to eliminate other light sources affecting the images. During the time needed for the leaves to make the starch, we can begin the paper chromatography activity described below. Before leaving class, the students will remove the leaves from the glass plates and soak the leaves in the hot alcohol bath. Past experience has shown that the leaves can be left in the alcohol bath overnight (with the heat source turned off!) and stained the next day.
Paper Chromatography
After a discussion about what pigments might be in chloroplasts, I have the students gather several types of leaves from trees outside the classroom. Since I am usually teaching this unit in the early fall, I tell them to collect some leaves that are starting to change colors, as well as some green leaves of the same type. I always have on hand some Southern Magnolia leaves. This tree is an evergreen, has very thick leaves, and always gives wonderful results in case other leaves have already started losing their chlorophyll. Paper chromatography is a method of separating molecules by solubility, size and their attraction to the paper. This lab is a modification of the AP Biology Lab 4, part A. Instead of preparing a solution of the leaf pigments, I have students use the edge of a coin to press the leaf pigments into the chromatography paper in a straight line across the bottom of the strip, about one half inch up. The pigment must be above the level of the solvent or it will all end up in the bottom of the jar. This method saves prep time and students can test a variety of different leaves. They must take care, however, to get a thick straight line of pigment on the paper, and not to tear through the paper with the coin. The strip should be about 2.5" by 4", and folded in half lengthwise. The students can put a line of pigment at the bottom of each half and the fold makes it stand up in the chromatography jar. (See Figure 3.) This method works best with actual chromatography paper rather than the coffee filters sometimes recommended as an alternative, because the thicker paper stands up better to being pressed by the coin. The strip is then placed in chromatography solvent—90% petroleum ether and 10 % acetone. Use a jar with a lid to contain the fumes. If you do not have these chemicals, alcohol or fingernail polish remover may work.
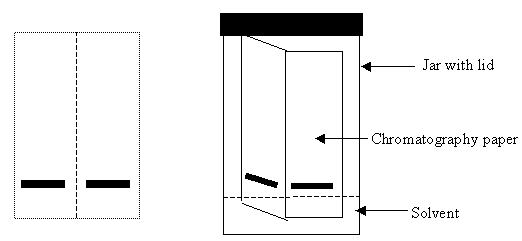
Figure 3. Chromatography lab setup.
As the solvent moves up the paper, it dissolves the pigments from the leaf and they begin to separate and move up the paper with the solvent. This only takes about ten minutes or so, depending on the solvent and the paper used. Because of the different properties of the pigments, they will move at various rates. Some will be more soluble, some will have a higher affinity for the paper fibers. Where students might have only seen green when they looked at a leaf, the chromatography paper will show other pigments that were in the leaf. Leaves that have changed colors will reveal a lack of chlorophyll. Chlorophyll a will show up as a bright Kelly green to bluish green. Chlorophyll b shows up as a yellowish green, usually below the chlorophyll a. Carotenoids will appear as pale yellow to an orange-yellow near the solvent front. Students should be able to identify the different pigments present in the leaves and measure the distance that each pigment migrated, compared to the solvent front. The ratio is known as the R f value of the pigment and can be used by the students to compare pigments from different species. Students record their observations, measurements and attach the chromatography paper into their lab notebooks.
Day 3—Light Reactions Lecture with Demonstration Model
Warm-up questions
What might plants require for photosynthesis? What might be produced during photosynthesis?
Students should be able to answer most of these questions based on what they have learned in previous science classes, or from what they have observed in the activities of the past two days. Or they could just make an educated guess. The wording of the questions is deliberate—"What might be produced?" I am assessing what my students may know about photosynthesis, but I don't want them to feel like there is just one answer that they have to know at this point.
Lecture and Demonstration Model
I then introduce students to the demonstration model of a chloroplast that I have built. (Instructions and plans are included in the Appendix.) I ask students what parts they might be able to identify based on the diagrams and activities they have done so far. They should be able to recognize the membranes of the chloroplast and perhaps some of the pigments based on the colors in the model. As I go over chloroplast structure, I will also use the "Fabric Thylakoid Demonstration" described below, and ask students to find similar layers in the model. I will refer to the components of the model as I proceed with a lecture on the light reactions. The background information on the light reactions given in this unit includes the references to the model I plan to use. Students will be given the two diagrams of the model from Figures 1 and 2 to make notes on. At the end of the lecture, I will have volunteers come up and manipulate the model to show how electrons flow from water to NADPH through PS II and PS I at different points during non-cyclic flow. The idea is that by having a three-dimensional structure that simulates as closely as possible how photosynthesis works in a chloroplast, that students will have a better grasp of what is going on.
Fabric Thylakoid Demonstration
To show students how the thylakoid membrane is folded to form grana and lumen and lamellae, use a 10 yard piece of fabric which has definite "right" and "wrong" sides. Identifying the "right" side of the fabric as the luminal side of the thylakoid membrane, fold the fabric back and forth to create grana and lamellae. (See Figure 4.) The "lumen" of the fabric thylakoids should have the "right" side of the fabric facing it. This demonstration shows how the thylakoids could be formed from just one or two membranes.
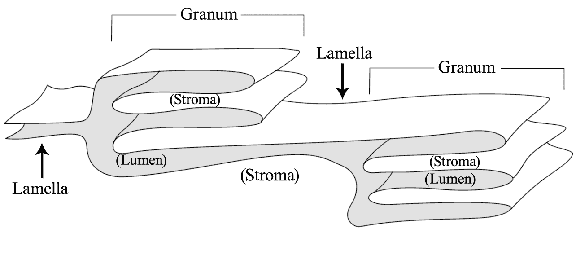
Figure 4 — Fabric thylakoid
Day 4—AP Lab 4B—The Light Reactions
Warm-up question
What role does NADP + play in the light reactions?
Photosynthesis Lab
In this AP lab, students test the effects of light and darkness, and boiling on the reduction of NADP by PS I in isolated spinach chloroplasts. The effects are measured by substituting a blue compound, DPIP (-2-6-dichlorophenol-indophenol), for NADP +. The DPIP becomes more and more colorless as it is reduced. This change in color can be measured with a spectrophotometer. The more DPIP that is reduced, the more photosynthesis that is taking place. An on-line version of this lab can be found at
http://www.ekcsk12.org/science/aplabreview/plantpigmentsandphotosynthesislab.htm
I begin this lab by showing students how to use the spectrophotometers. I then ask the class to use the demonstration model to explain what the DPIP is supposed to do in this lab. After completing the lab, students are given additional questions that relate the lab results to the demonstration model:
- Does isolation of the chloroplasts affect the light reactions? Why or why not?
- Describe what is happening in the model when the chloroplasts are exposed to light.
- What happens when they are placed in the dark?
- Show on the diagram of the model where the DPIP would come into play.
- What does changing the length of exposure to the light do in the thylakoids?
- What would be happening in the model if the chloroplasts are boiled?
The idea is for them to relate what is happening in the cuvette to what they can see in the model.
Day 5—Calvin Cycle Lecture and Role-play
Warm-up questions
Where might the O 2 come from in photosynthesis? What else is made at the end of photosynthesis? How does CO 2 get into the chloroplast? How does water get into the chloroplast?
The Kool-Aid Cycle
Objective: Students will discover how cycles such as the Calvin cycle work and how some components leave the cycle and some are maintained in the cycle.
Materials: large plastic cups, plastic spoons, 2 quart pitchers or other container, Powdered, unsweetened Kool-Aid in pre-measured 0.5 g amounts, sugar pre-measured in 1/8 cup portions, water, measuring cups.
Procedure:
- Set up stations around the room for each of the following items: 2 Kool-Aid stations, 2 sugar stations, 2 water stations, 3 spoon stations, one pitcher station with several pitchers, 2 "dishwashing" stations. Like stations should not be next to each other if possible. Assign one student to man each station except the pitcher station. If the class is small, you can combine tasks at a station, but there must be at least 2 of each station except the pitcher station.
- Divide the rest of the students into teams. Give these students plastic drinking cups. Their task is to make enough Kool-Aid to fill up a 2-quart pitcher. The problem is they can only make one cup at a time, and they must go to the different stations, in order, to get the job done. They do not have to go to the same stations each time. If someone else is being served, then the student has to wait or go to the other station for that part of the cycle. (If the class is small, you could omit creating teams and just time them on how long it takes to fill one pitcher, then discuss what happened and if they can think of ways the process could be speeded up without breaking the rules.)
- Each student with a cup must first go to a Kool-Aid station where the person manning that station will pour the aliquot of powdered Kool-Aid into the cup.
- Next the student with the cup must go to a sugar station. Again, the person manning that station will distribute the sugar into the cup.
- Next is a water station. Here they will receive 1 cup of water, measured out by the person manning that station.
- Then on to a spoon station, where the person manning that station will stir the Kool-Aid until it dissolves
- With the final product complete, the student may now visit the pitcher station and pour their Kool-Aid into a pitcher.
- Before they can begin the cycle over again, they must visit a dishwashing station where they will have their cup rinsed out by the person at that station.
- Finally, they are ready to begin the cycle again, continuing until they have filled their group's pitcher.
- Have everyone fill a cup with Kool-Aid to drink and discuss the following questions.
Questions for Discussion:
- How was this activity like a cycle?
- Describe how the students with the cups moved around during this activity?
- What might the people manning the stations represent?
- What were the reactants and products of this activity?
- What was not changed in the activity?
- Was anything recycled? How?
- What station got backed up the most? How could this be prevented?
- How many times did the cycle have to occur before the pitcher was full?
- What might happen to the cycle if all the pitchers were filled and no one wanted to drink anymore Kool-Aid?
The Calvin Cycle Sashay
Students are given an outline of the Calvin cycle to complete after we act out the steps in the form of a square dance. As this interactive "lecture" on the Calvin Cycle proceeds, students are given roles to play as each molecule enters the cycle. Depending on the number of students, you may have to adjust the number of characters that are represented.
Following the dance, we will discuss how the dance related to the Kool-Aid activity and how it compares to a textbook diagram of the Calvin cycle.
One student, or the teacher, will be Calvin Rubisco and call the dance. Calvin, of course, is the enzyme who gets this whole thing started by bringing the dancers together. During the first part of the dance there should be 3 couples—Ruby Pea (RuBP) and Carbon Dioxide in each couple. Later on you will need 2-6 students to be ATP, and 2-6 to be NADPH. (Of course, these can be recycled, too!) The Rubys each hold 5 Styrofoam "carbon" balls connected with toothpicks, with a ball labeled as a phosphate group on each end.
Each Carbon Dioxide just holds one of the large Styrofoam balls to represent CO 2.
Changes in names are denoted during the dance by flipping a set of nametags that hang around the dancers' necks.
- 3 Sets: RuBP—PGA—GBP—G3P—RuBP
- 1 Set: CO 2—PGA—GBP—G3P(sugar)
- 2 Sets: CO 2—PGA—GBP—G3P—(sit down)
- ATP Sets: ATP—ADP
- NADPH Sets: NADPH—NADP+
Walk students slowly through the dance at first, until they have worked out the movements. Then try it with square dance music. Let the music play a bit between calls if students need time to put things together.
The dance begins with the three couples in a circle. Rubisco calls the dance:
Chorus 1: Circle left all day long, we'll make sugar from dusk til dawn.
Circle-cycle to the right, we'll even make sugar into the night.
(Dancers circle left, then right)
Allamande left, allemande right, Ruby find a partner and hang on tight.
(Ruby and CO 2 dancers walk in opposite directions in a circle, alternating each other on the right and left hand sides, until they meet their partner again.)
Ruby and Carbon one day meet, combine their carbons, ain't that sweet!
Put your carbons all together, 6 for now, but they won't weather.
(Here the Ruby and CO 2 partners attach all their balls together to form a 6-carbon compound.)
Chorus 1: Circle left all day long, we'll make sugar from dusk til dawn.
Circle-cycle to the right, we'll even make sugar into the night.
(Couples circle left and then right, holding their new molecule between them.)
Share your carbons equally. Once was 6 C's, now there's 3.
(Couples split apart the 6-carbon compound and form two 3-carbon compounds, each with one phosphate attached. Each partner holds one of the new PGA molecules)
Chorus 2: Flip your card, you're something new.
Remember what you have to do!
(Couples flip their name cards to PGA)
Pretty good allies, PGA, but you'll go your separate ways.
Chorus 1: Circle left all day long, we'll make sugar from dusk til dawn.
Circle-cycle to the right, we'll even make sugar into the night.
(All dancers circle left and right)
Now new dancers come on in, with energy to make us spin!
(ATP dancers enter the circle with "phosphate" balls)
A-T-P has en-er-gy, to give to you, with a "P"
(ATP dancers attach a phosphate to each PGA dancer's molecule.)
Chorus 2: Flip your card, you're something new.
Remember what you have to do!
(PGA dancers flip their cards to GBP after receiving a phosphate from ATP. ATP flips to ADP and moves off the dance floor.)
GBP, you're looking fine, with 2 phosphates, you're divine!
Now new dancers come on in, with more energy to make us spin!
(NADPH dancers enter the circle, each with a hydrogen and 2 electron balls)
Chorus 1: Circle left all day long, we'll make sugar from dusk til dawn.
Circle-cycle to the right, we'll even make sugar into the night.
(All dancers circle left and right)
N-A-D-P-H gives you, a proton and electrons—2!
(NADPH dancers attach a proton and 2 electrons to the GBP dancers' molecules.)
Re-duced now and ain't that great, Lets get rid of 1 phosphate.
(GBP dancers throw one of their phosphates into the air, and off the dance floor. ADP dancers can pick them up.)
Chorus 2 : Flip your card, you're something new.
Remember what you have to do!
(GBP dancers flip their cards to G3P, NADPH flips to NADP+ and moves off the dance floor.)
Chorus 1: Circle left all day long, we'll make sugar from dusk til dawn.
Circle-cycle to the right, we'll even make sugar into the night.
(G3P dancers circle left, then right.)
Now there are six G3P's, five must stay and one must leave.
Turn and wave bye-bye for now. S(H)e'll be sugar anyhow!
(One of the dancers leaves the dance floor. The other 5 wave good bye.)
G3Ps you are so sweet, but our cycle's not complete.
In the center all join in, to put 5 carbons back again.
You'll need the help of an old friend, 3 ATPs are here to lend.
(The 5 G3P dancers come together and pull apart and reform their balls, along with 3 phosphates from the ATP dancers to make 3 RuBP molecules.)
Chorus 2: Flip your card, you're something new.
Remember what you have to do!
(Three of the G3P dancers flip to RuBP and hold the new molecules. Two dancers flip to a "Sit down" card. ATP flips to ADP)
All the RuBPs circle right, Three with 5 C's—you did just right!
This circle dance begins again, when 3 more carbons come on in!
Day 6—C4 and CAM Plants
Warm-up questions
What might happen if rubisco could also bind with O 2? How might living in the desert affect photosynthesis in a plant?
Models of C4 and CAM plants
Students will be given notes on the adaptations of these types of plants based on the background information included in this unit. They will then have a choice of completing one of the following two assignments:
- Take our skit for the Calvin cycle and revise it, or create a whole new skit, to demonstrate what happens in either C4 or CAM plants. Present your skit to the class.
- Design a plant with adaptations that allow it to carry out photosynthesis in some extreme environment. You must address new ways of carrying out either the light reactions or the Calvin cycle, as well as any specialized structures that the plant would need.

Comments: