Background
Food is a complex of natural and synthesized molecules often carrying a diverse set of deliberate chemical additives including flavors, coloring agents, preservatives, stabilizers, emulsifiers, fragrances, drug residues, and plastics that migrate from packaging materials.7 Food is always a great part of our lives. In the human body, it provides nutrients for energy, growth, repair, and regulation of body processes. But because of its complexity, it sometimes becomes a subject of concern. Numerous research studies have shown that cancer and other forms of diseases are related to different chemicals found in food. Disorders are perceived to be caused by chemical exposures from changing environment conditions brought about by nearly 100,000 molecules traded in the world market.8 These chemical exposures can virtually affect every cell in the body. They can affect enzymes, the proteins that regulate many important chemical reactions. These can also lead to harmful substances binding directly to the cells, or to molecules within the cell like carbon monoxide binding to blood hemoglobin. Food chemicals like carbon tetrachloride (CCl 4) can stimulate large release of epinephrine from the nerve cell that can cause liver damage. These harmful substances can have delayed or long-term effects. Effects are not only shown in humans. They can also be seen in our environment.
Food chemicals and packages have adverse effects to the environment. They proliferate in wild life-encroaching landfills scattered around the country. In Chicago alone, there are 10 major landfills. 23% of 2.5 million tons of trash collected from these landfills in 2003 were mostly from foods and plastics that cannot be recycled.9 Toxic substances from plastics leach to fresh and marine water sources. Aquatic animals also eat plastic materials. This leads to disturbance of ecological balance. This directly or indirectly harms the food chain when toxic substances accumulate in living organisms and increase contamination levels on different kinds of species.
Polymers
Food molecules exist in the form of polymers. Polymers are made up of many molecules all strung together to form long chains. These long chains are made up of smaller units referred to as monomers. Various kinds of monomers form the two kinds of polymers, natural and synthetic. Natural polymers include proteins, lipids, and polysaccharides (the biomolecules in food). Synthetic polymers include silicone, plastics, nylon and epoxies. Polymers are very interesting because it is the kinds of molecules that dictate the properties. Adding "stick-in molecules" can alter the strength of the polymer. Plastics, for instance, become stronger by adding plasticizers.
How are natural polymers formed? In living cells, polymerization starts with elements carbon, oxygen, hydrogen, nitrogen, and sulfur. These elements are initially utilized to form small number of building blocks (amino acids, glucose, acetyl co-A and nucleotides). These building blocks are in turn used in the construction of a vast array of vital organic biomolecules (nucleic acids, polysaccharides, proteins and fats). Figure 1 shows how biomolecules are formed in living cells.
(Polymerization in Cells)10
Figure 1
Synthetic polymers can be formed just like natural polymers. Polymerization is done in two ways: by addition or by condensation.
Addition polymerization is a polymer reaction where the entire monomer becomes part of the polymer:
Addition Polymerization: CH 2=CH 2 + CH 2=CH 2 -> CH 2=CH-CH 2-CH 3
Condensation polymerization is when a small molecule is given off as the monomers combine. In the example below, the small molecule is water.
O
R - C - OH + H - OR" -> R - C - OR" + H 2O
Carboxylic acid Alcohol Ester Water
Biomolecules
Carbohydrates are the major sources of energy. These include sugars, starch, and cellulose. These are made up of carbon ©, hydrogen (H), and oxygen (O) in the ratio of 1:2:1. Carbohydrates may be monosaccharides, or simple sugars, such as glucose and fructose; they may be disaccharides or polysaccharides where two or more monosaccharides form chains. Monosaccharide contains one basic sugar unit, glucose. It is the major carbohydrate found in plants and animals. Glucose is represented by a cyclic structure with the molecular formula C 6H 1 2O 6. Below are the two different isomers of glucose: fructose and galactose.
Figure 2: Structural Formula of Fructose 11
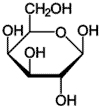
Figure 3: Structural Formula of Galactose 12
Starch and cellulose are examples of carbohydrates with many sugar units called polysaccharides. While starch can be digested into glucose, which serves as a source of energy, cellulose cannot be digested. Both starch and sugar are natural polymers of glucose. Starch is stored in fruits, roots, and seeds of plants. Glycogen, an animal starch, is stored in the liver and muscles. Cellulose, on the other hand, is the principal structure of plant cells and fibers. The building material in cellulose cannot be consumed for energy due to its different structural arrangements as compared to starch.
The presence of sugar in food can be tested with Benedict's solution. The color changes from blue to reddish orange, the latter indicating greater amount of sugar. Staining by Iodine solution distinguishes starch from sugars. The basis of this test is that starch is a coiled polymer of glucose and that iodine interacts with these coiled molecules and becomes bluish black. A yellowish brown color is a negative test for starch.
Whenever we eat meat, fish, cheese, or eggs, and drink milk, we supply ourselves of proteins. Proteins are remarkably versatile molecules for growth and development and are found in all life forms. Proteins are composed of monomers of amino acids with amino and carboxyl groups. The amino group (NH 2) on one amino acid is linked to the carboxyl group (COOH) on an adjacent amino acid by a peptide bond.
Figure 4: Amino Acid Structure 13
The peptide bond forms through dehydration synthesis. Dehydration synthesis involves two monomers (amino acids) of proteins that join together and one of the products released is water. This peptide bond is the site of action for the Biuret test for proteins. The Biuret solution is light blue, but in the presence of proteins, it turns violet, the intensity of the color relates to the number of peptide bonds that react. Long chains of polypeptides (which is also a polymer of proteins) have many peptide bonds and produce the most positive reaction. Free amino acids and very short chains may result in a pinkish color.
Lipids are groups of molecules including fats and oils, waxes, phospholipids, steroids and some other related compounds. All lipids are hydrophobic(water-hating). Fats and oils are made from two different kinds of molecules: glycerol and fatty acids. The Sudan IV test is a useful laboratory test for fats. If fats and oils are present, these will appear as floating red droplets or as a floating red layer colored by Sudan IV. Fats are present in almost every food.14 Milk, eggs, meat, and other animal products contain fats. Whole grain cereals and oatmeal range in fat content from 1% to 7%. All types of nuts contain about 70% fats. Fats are also considered sources of energy. They can sustain life for about five weeks provided water is available.15 However, excess fats are not good for the heart. The extra load causes strain on the heart as it pumps blood to different parts of the body. The smallest unit of fats is fatty acids. The structure is given below:
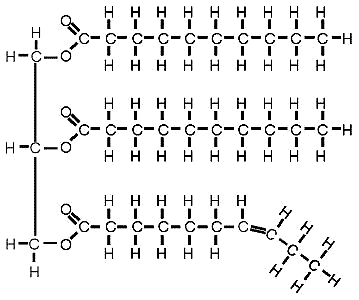
Figure 5: Fatty Acid Structure 16
Food Additives
A food additive is any substance that is used to condition food as a dietary supplement and to affect some useful purpose in the food. It may also be any substance that affects the characteristics of foods.17 The use of food additives has been practiced for thousands of years. The common household additives are vinegar, salt, spices, and sugars. Some additives are used to retard spoilage, some to retain freshness, enhance flavor and increase the attractiveness and acceptability of foods. In food processing, additives are used to sanitize, bleach, and improve food texture. Almost all instant foods contain a variety of food additives.
An additive may have two functions. Spices and vinegar for example preserve the food and at the same time improve the flavor. Some household additives include sodium citrate, sodium phosphate, and monosodium glutamate (MSG). In using any food additive, its dose or quantity should be a great consideration due to the harmful effects it may cause. Monosodium glutamate (MSG), when used in large quantities, produces profuse sweating and dizziness. It also causes severe headaches even at small quantities among sensitive individuals. Food additives that are considered dangerous are those that are introduced in food through the use of insecticides like DDT and antibiotics such as streptomycin.18 Carcinogenic additives are those that cause cancer. These carcinogens may come from cooking styles. Broiling with charcoal can give benzopyrene, a strong toxin, to the steak. Benzopyrene comes from the fumes of charcoal. Another example of carcinogenic additive is safrole. Safrole is commonly found in root beer.19
Foods contain preservatives. They prevent spoilage and give long life to products. They are widely used by food processing industries and they are mostly found in soft drinks. One example is sodium benzoate that is currently a subject of concern about cancer. If we mix this substance with vitamin C additive in soft drinks, benzene is formed. Benzene is a carcinogenic substance that causes decrease in red blood cells and damages the bone marrow and the immune system.20 Studies show that benzene is also linked to some genetic diseases like Parkinson's Disease because this compound has the ability to destroy mitochondrial DNA in cells that triggers cells to malfunction.21
Table salt is one of the earliest food preservatives. It can retard the growth of microorganisms. It also regulates the growth of fermentation. It is widely used for cooking because salt is an excellent binder. It binds with meat, fish, and other foods and thus increases the solubility of these muscled substances in water. Adding salt also gives taste to food, and consequently, we get pleasure from eating. In addition, salt provides our body with minerals like sodium and chlorine. But increased amount of salt in food is not healthy.
Hypertension is directly linked to too much salt. It is also associated with other forms of health problems. Gastric cancer was found related to dietary factors like the intake of salt and salted foods. It does not only increase the risk of Helicobacter pylori infection, it also adds to the risk of gastric cancer.22 Adverse effects of abnormal salt intake include risk of stroke, renal diseases, asthma, cirrhosis, heart failure, and stomach cancer. Since most salt consumed comes from processed foods, steps must be taken to decrease the amount of processed food intake.
Food coloring, stabilizers and flavors are also added to foods. Colorings are there for aesthetic reasons, to make food attractive and appealing. Stabilizers are thickeners that give food a firmer texture and viscosity. Flavors are not altogether safe. Adding artificial sweetener is associated to teeth decay and erosion, abnormal weight gain, depression, anxiety, sleep difficulties, headaches, increased appetite and seizures. Abnormal weight gain, for example, results from having the body crave for more food when sweetener signals the body to store carbohydrates and fats. Once glucose levels decrease, the person feels lethargic and the feeling of hunger kicks in.23 This can lead to increased eating.
Pesticides
Pesticides grew with human population. More food was needed to feed the increasing number of people. Plants as sources of food need to be protected from pests. The use of pesticides became a popular means to control or eliminate pests. But pesticides when applied to plants often persist as residues. A recent analysis of non-organic food found that 73% of samples contained pesticide residues. Tests of a single vegetable found ten different pesticides while several single pieces of fruit contained eight.24 Imported foods usually contain pesticide residues and are marketed without any warning to consumers or penalty to importers.25
Children often are more susceptible to pesticides; their diets are exposed to pesticide residue 10 to 20 times the average levels experienced by adults.26 Food intake is highest during this growing stage. They are most likely to suffer because of their lower body masses. Moreover, their immune systems are not yet well developed. They get more pesticide exposures from homes, yards, schools, parks, or even from playgrounds. When they play, we let them drink from garden hose or spray them with water from it.
In consequence, the government thru the EPA reviews studies on pesticides so that its use will not pose unreasonable risks to human health and the environment. EPA sets a tolerance, or the residue level that triggers enforcement actions. If residues are found above that level, the commodity will be subject to seizure by the government. Tolerance levels are published in the Federal Register.27
Examples of commonly used pesticides include Boric Acid, Chlordane, Diazinon and Hydropene. These are proven effective against insects, spiders, algae, molds, fungi, weeds, and termites. Some pesticides are used as antimicrobial or antiviral agents. Examples of these are the Accel TB for Myobacterium tuberculosis, 25 RTU for Human HIV -1 Virus and the 65 Disinfecting Heavy Duty Acid Bathroom Cleaner for MRSA.28 The National Pesticide Information Center gives a list of government-approved pesticides including their chemical ingredients, uses, and toxicity levels.29
Plasticizers
Polychlorinated biphenyls or PCBs are examples of poisonous compounds that have varied uses. These are used as components of adhesives, asphalt roofing material, dyes, fluorescent light ballasts, inks, lubricants, paints, carbonless copy paper, pesticides and rubberizers.30 PCBs are also used in the manufacture of different plastic containers. In some countries, food contaminated with PCBs were found to cause hair loss, enlargement of the liver, and disorders of the intestinal and lymphatic systems.31 They do not easily decompose and are suspected to be carcinogenic. They are found to be soluble in fats of animals and are stored in human tissues.32
Production of all kinds of commodities has increased exponentially and to preserve these materials, the use of plastics proliferated. Plastics have two components, Bisphenol A and DEHP. Both substances are believed to cause changes in growth and development of many species of animals. Bisphenol A is a white to light brown flakes or powder substance that has a chemical formula C 1 5H 1 6O 2 or (CH 3) 2C(C 6H 4OH) 2.33 BPA is a chemical compound with high molecular weight, melting point, boiling point, and is water-insoluble.34 It was invented about 120 years ago. This substance is used an important chemical building block to make polycarbonate plastics and epoxy raisins, both of which are believed to serve in a wide variety of applications that were intended to make our lives better and safer.35 It has been utilized extensively. About 40 years later, its first evidence of toxicity was revealed. Now, it is known to contaminate 93% of the population.36
Sources and Health Effects Related to BPA
BPA is now deeply embedded in the products of modern consumer society. It is used to form pesticides, antioxidants, flame retardants, rubber chemicals and polyvinyl stabilizers. We are also exposed to it through films, pipes, computers, refrigerator shelvings, microwave ovens, floorings, enamels, varnishes and adhesives, automobile parts, water bottles, baby bottles, and eating utensils. We can see BPA in plastics everywhere, from children's toys to our drinking cups, from cosmetic products to baby diapers.37
BPA is a hormone-disrupting chemical considered to be potentially harmful to health and the environment. In different studies, BPA was found to mimic the female hormone estrogen in test animals and some marine organisms that led to the discovery that female sex organs have sprouted in some male fish and seagulls. BPA is also very much present in ground and surface water. If pesticides travel from crops to drinking water supplies in two years, then BPA must be doing the same thing. The widespread use of BPA in consumer products and its presence in environmental media have lead to the detection of BPA in human urine, serum, breast milk, maternal and fetal plasma, amniotic fluid, and placental tissue at birth. Though BPA has no clear effects on humans, as as the American Chemical Council has found out, studies have shown adverse health effects on some test animals. These include meiotic failure, reduced sperm count, mammary gland development, prostate disease and cancer, diabetes and obesity, impaired human function, behavioral changes, and brain effects. BPA has been shown to have physiological effects in development of tissues of these organisms both in vitro and in vivo. In Japan, women with history of miscarriage were found to have high levels of BPA. With uncontrolled use of BPA, researchers, scientists, and educators started to study its effects on human health and environment.38
Government researchers and agencies are aware of BPA's persistence under normal conditions in the environment. It does not readily degrade. Yet, there are no laws set to regulate BPA in the market. BPA shows no matter how proofs of toxicity even at very low doses persist. Government agencies like EPA provides safety standards which are at odds with scientific results. Thus, people continue to use them. In the United States alone, the estimated consumption of BPA-containing plastic is 223 poinds/person/year. It is also estimated that consumption will increase to 300 pounds/person/year by 2010.39
Life is easier with plastics but we suffer some serious consequences. Marine and aquatic ecosystems are affected by having planktons and other organisms feed on plastic and its components. Therefore, we can say that we do not only get plastic-contaminated foods, we are also taking these toxic substances through the food chain.
DEHP, Its Sources and Health Effects
Another component of plastic is diethylyhexylphthalate or DEHP. It is one of the most cost effective and widely used general purpose plasticizer. It is used mainly for making PVC soft and pliable.40 It is a compound that can easily bind with anything. DEHP has a chemical formula of C 2 4H 3 8O 4.41 It is a colorless almost odorless liquid that is insoluble in water, miscible with mineral oil, and soluble in most organic solvents. In the atmosphere, it is present as a gas or attached to solid paticles. During precipitation and rain, it can persist in the environment. If a DEHP-containing material is thrown as thrash and ended in landfills, this chemical can leach to grounwater. Contamination with DEHP can come from many different sources. Phthalates are present in all food and packaging materials, medical devices and products, flexible tubings, electrical conduits, building products, lubricants, perfumes, hairsprays, cosmetics,construction materials, wood-finishers, and adhesives. The probability of exposure especially among children is high because most PVC applications like teethers and toys contain DEHP.
DEHP, just like BPA, is known to have greatest potential among phthalates to cause diseases and this is more potent at low doses. Effects on animals were on male reproduction, brain development, early onset of puberty in males, delayed/advanced puberty in females, and allergies.42 Studies show that it is found in human blood, seminal fluid, breast milk, and saliva. Human studies were also conducted and phthalate exposures also resulted to male reproductive malformation, sperm damage, asthma, early puberty in girls, female reproductive tract diseases, premature delivery and thyroid effects.43
The Food Pyramid Guide
The pyramid in Figure 7 is composed of six food groups. Although it is important to eat foods from all the food groups, the pyramid also gives information on the number of servings per day. For good health, fats, oils, and sweets at the very top should represent only a small portion of a diet. It is recommended that daily food choices must come from the bottom section (6 to 11 servings) and the middle groups (3 to 5 servings).
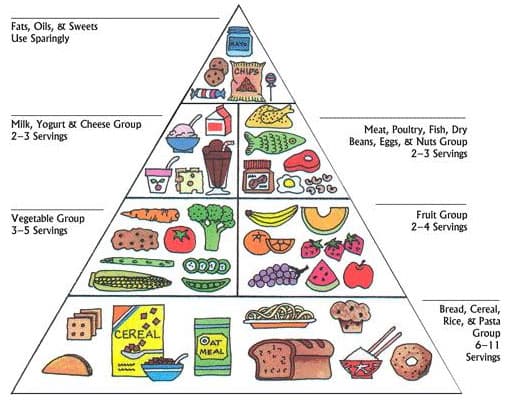
The Food Pyramid Guide 44
Figure 7
Label Statements on Foods
FDA states that required label information must be conspicuously displayed in terms that ordinary consumers like us can easily read and understand. Package labels should include the manufacturer's name, address, packer, and distributor. It should state an accurate statement of net amount of food in terms of weight, measure, or numerical count.45 The ingredients in food should be listed by their common names in decreasing order by weight. Food additives are required to be listed as ingredients. Artificial colors and flavors should be named specifically. Nutrition requirement should be provided. If the food labels bear representations in a foreign laguage, they should be translated into english. The government, along with its agencies, is trying its best to assure safety and proper labeling for human consumption, but because of limited federal funding, these agencies like Centers for Disease and Control (CDC), Food and Drug administration (FDA), and United States Department of Agriculture (USDA) can only do as much.
Reported occurrences of outbreaks of food-borne diseases have been increasing, and current safety efforts are not providing the confidence in the food supply that U.S. consumers demand.46 Another concern that we have to think about is that usually, foods are only labeled if they meet certification standards. Some organic products sold in the market are properly labelled but majority are not.
It is important that students and readers understand the most common names and symbols often used for labeling plastics especially for classifying and recycling purposes. The following is a list of plastic symbols, numbers, names, and sources:47
- PETE ( Polyethylene Terephthalate) - is a thermoplastic resin used in synthetic fibers, beverage, food, and other liquid containers.
- HDPE (High Density Polyethylene)-is made from petroleum and used in milk jugs, trash bags, ice cube trays, andstorage containers.
- PVC or V poly (Polyvinyl Chloride, DEHP)- is a thermoplastic polymer widely used in construction materials, cooking oil bottles, baby bottle nipples, and packaging around meat.
- LDPE (Low Density Polyethylene)- is thermoplastic made from oil and used widely in manufacturing containers, dispensing bottles, wash bottles, tubings, plastic bags for computer components and various molded lab equipments. The most common term is plastic bag.
- PP (Polypropylene)-used as margarine tubs, yogurt containers, straws, spice containers, and trays for microwaveable meals.
- PS (Polysterene)-is a thermoplastic made from petroleum. This is used in styrofoam cups and containers, take-home boxes, egg cartons, meat trays, plastic model kits, license plate numbers, plastic cutlery, and CD containers.
- Others (Bisphenol A) -which means the plastic is not made up of the initial six or some unique combination of the initial six. These are found in polycarbonate baby bottles, water cooler bottles, and toodler fruit cups.

Comments: