Birth: the primordial ooze to the chemistry of bottle life
Most plastic bottles are made from products produced from natural gas and the distillates of petroleum. So, the story of the plastic bottle necessarily starts with petroleum and the distillation process. The US Department of energy has a good website explaining how fossil fuels form. 2 Many good Earth science textbooks will also have an explanation of fossil fuel formation. What follows is a brief version of the story of fossil fuel formation, followed by an explanation of distillation and concluded with a description of the chemistry involved in making PET. A very brief explanation of organic chemistry is also included for those of us who haven't had organic chemistry in a while. The primordial swamp to fossil fuels Life began on Earth several billion years ago, precisely how many billion years ago is still being debated, but it was probably between 3 and 4 billion years ago. A long time after life first began, plants began photosynthesizing, capturing sunlight and using the energy to transform carbon dioxide into carbon chains, which became sugars, starches, and plant fibers. Some of these plants formed the basis of a food chain which supported a variety of living organisms. As these plants and other living things died, they did not immediately decay. The normal, natural processes of decay allow carbon and other nutrients to be recycled back into an ecosystem. However, in the case of these plants and animals, there was little or no immediate decay. Instead the starches, sugars, and fibers from the plants and tissues from the animals that may have died with them, broke down slowly and the carbon was not cycled back into the ecosystems. Over time land formed on top of the dead organisms and began to compact them. Compaction causes both temperature and pressure to rise, which caused the carbon chains to change from being fibrous structures to being a fossil fuel. A similar change, although less extreme, can occur when plant matter, like veggies, are left in a pressure cooker for too long. The vegetables change from being crisp and fairly rigid, to being soft and gooey, although still identifiably vegetable. The formation of fossil fuels takes much more time, pressure and heat.
Different circumstances formed different types of fossil fuels. Today there are three major sources of fossil fuels: coal, crude oil, and natural gas. Coal was formed when swamps were buried. This formation is still happening in the early stages in peat bogs around the world today, which is why peat makes such a good fuel. Oil and natural gas formed when deceased organisms sank to the bottom of the ocean. The organic mater was slowly covered and compressed. More compression leads to smaller carbon chains, forming natural gas. Less compression allowed longer carbon chains to stay together, so crude oil was formed. Small pockets of oil and gas formed in sedimentary rock. Under pressure, the oil and gas were forced up through the fairly porous sedimentary rock. In some cases, the oil and natural gas simply seeps to the surface. In many cases, however, the oil or gas was stopped on the way to the surface by an impervious layer of rock, which trapped the fossil fuels and allowed larger deposits to build up. Wells are drilled through the impervious rock and oil and gas are pumped out of the deposits. This is where most of our oil and natural gas come from today.
Class activity: history of life on Earth
In New Haven geology is taught in both the eighth and ninth grades. In the ninth grade, geological cycles are not taught until after this unit, so we will probably do a short introduction to the subject during which we will discuss how the petroleum and natural gas are formed. There are a number of interesting activities which can be used to teach this topic. I will probably use one that is fairly short, however, because we will be studying this topic in depth later during the year. What I most want my students to get out of this activity is the idea that fossil fuels are formed over millions of years, which is why they are not readily renewable. However, many students, particularly in the ninth grade, do not have a good concept of what "millions of years ago" really means. This activity is designed to give the students some idea of the relative magnitude of the geological time scale.
The activity I intend to do with my students is a variation on an activity I came across several years ago, whose original author I have yet to identify. The students will be asked to trace their arm onto a piece of long paper, possibly a piece of brown paper towel. They may need a friend to help them with this activity. It is important that when the arm is traced, the hand is flat on the paper, so it can be traced as well. The arm and hand will form the basis of the geological time scale. The students will label the arm with geological events. So, the shoulder will be about 4.6 billion years ago, when the Earth was forming. The biceps will be about 3 billion years ago, when the first life was appearing. The elbow is about 2 billion years ago. The middle of the forearm is about 1 billion years ago. The base of the thumb is the beginning of the Cambrian period. The base of the fingers is about 250 million years ago, or about the end of the Permian period, so much of the oil, gas and coal formation happened during the time represented by the palm. The space between the base of the fingers and the third knuckle is when the dinosaurs lived. About 1/8 of an inch from the tip of the finger, the glaciers receded from Connecticut. About 1/200 of an inch from the tip of the finger, the pyramids in Egypt were being built. This activity might also be a good opportunity to introduce students to the logarithmic scale, too.
Petroleum cracking
Crude oil is the source of most of the chemicals used to make plastics. For some plastics, like polyethylene and polyethylene terephthalate, natural gas or methane can also be a source of component monomers. However, nearly all plastics use some form of petroleum distillate, like ethylene glycol at some point in their formation, and ethylene glycol is frequently used in PET production. 3 As noted above, crude oil is the remains of organic matter that lived many millions of years ago. Because it is formed from partially decayed or undecayed matter, the carbon chains have denatured and ended up in hydrocarbons of many different lengths. Petroleum cracking is the way that the different component parts, or the different hydrocarbon chains, are separated from the mess that is crude oil. I may do a demonstration here using different lengths of something like spaghetti to symbolize the different carbon chain lengths and have the girls try to sort the pasta by length.
At this point in the year, the class will already have discussed phase changes and will have done an experiment on evaporation and condensation; ideally, we will have done one making distilled water; at the very least we will have done an experiment boiling colored water and watching the steam condense on a cooler surface, like a mirror. At any rate, the students should have a fairly good understanding of the idea that liquids can evaporate and recondense, and that they condensate may be somewhat different from the original evaporated liquid.
Petroleum cracking is based on the idea that every chemical volatilizes, or evaporates, at a different temperature. In the same way that grain alcohol is distilled from a fermented mash, petroleum distillates are distilled from crude oil. The primary difference is that when grain alcohol is distilled, there is generally only one particular distillate that the distiller is looking to capture; the rest is waste. In petroleum distillation, there are many compounds which the distillers wish to capture, so the distillation occurs using many different temperatures, rather than just one, as in alcohol distillation.
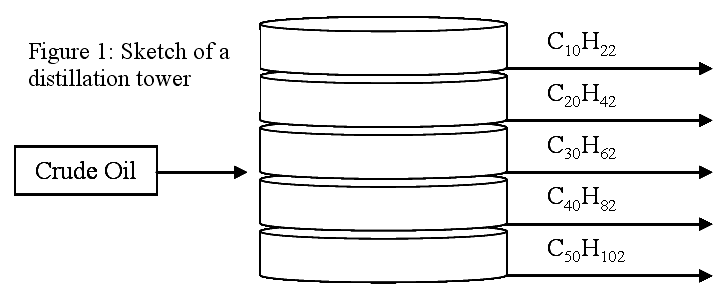
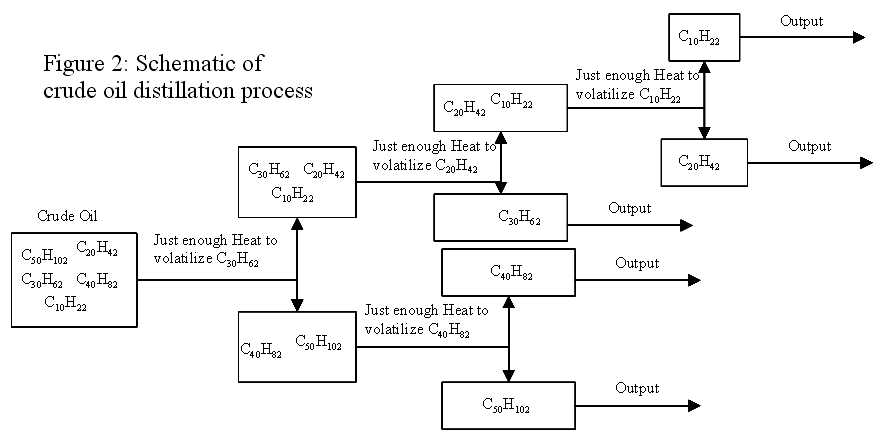
Crude oil is allowed to enter a distillation column. Heat is applied and some of the shorter polymer chains inherent in crude oil volatilize and rise up through the column until they meet a tray that is cool enough that they condense. The longer polymer chains are separated in a similar fashion further down the column. Each tray is at a different temperature, so different molecules will condense on it. From each tray, the condensate is removed and sometimes further refined, as needed. The heaviest, longer chains generally do not volatilize and remain as a liquid or solid, which is moved to another location. Figure 1 shows a schematic of a distillation column, with its trays. This is a very simplified version of a very complex process, so only a few of the compounds present in crude oil are shown. Figure 2 is a schematic showing the separation process that occurs in distillation towers.
Organic Chemistry
Organic chemistry is the study of molecules that contain carbon, generally large numbers of carbon atoms. Like many scientific fields, it has a vocabulary and language of its own. Unlike many scientific fields, the vocabulary in organic chemistry, and plastics manufacture in particular, tends to be unique to the field, so it really requires some explanation before use. When I teach a subject which requires unusual vocabulary, I generally start with the vocabulary, so that we have a common language to use for discussions. What follows is a brief description of several terms that are commonly used in the plastics industry. When I teach this section, I will give the students the words on individual scraps of paper and the definitions on another and have the girls match the word with its definition. Many of these words have great potential for decoding activities, which I tend to use in my class. I find that students have a better chance at remembering words, if they can make a connection to some of the roots or other bits and pieces of the words.
- Monomer: a single molecule which can be linked to others of its kind to form a polymer.
- Polymer: a collection of monomers, generally in the form of a chain, although sometimes branching or forked. Polymers can be natural, synthetic, or synthetic derived from natural materials. Hair, proteins, cotton fiber, silk, wool, wood, and fur, are just a few examples of natural polymers. Rayon is an example of a synthetic polymer that is made from natural polymer materials. Nylon, polyester, bakelite, and high density polyethylene are examples of synthetic polymers.
- Resin: a collection of polymer molecules that have the potential to become a plastic.
- Plastic: a synthetic long chain synthetic polymer that has been formed or molded in some way. 4
- Plasticizer: a chemical added to plastics polymers used to help the polymer chains slide past each other during the formation of the plastic and sometimes its subsequent life. Plasticizers act like ball bearings act in many mechanical applications, in that plasticizers unattached to the polymer molecules and allow the polymers to slide past each other. Like ball bearings, plasticizers are frequently not attached to the objects or molecules whose motion they are intended to ease.
- Amorphous: A solid state of plastics in which the polymers are tangled and disorganized, similar to spaghetti. 5
- Crystalline: A solid state of plastics in which some of the polymers are organized and oriented in a similar direction. 6
- Thermoplastic: A plastic which is solid at room temperature, can be heated, shaped, and cooled to create a desired shape. 7
- Condensation: A polymerization reaction releasing water
Polymer formation begins with the addition of monomers to form small chains. Many monomers are actually gasses as the molecules tend to be very small and somewhat volatile. The small chains of monomers, called oligomers, are sometimes still gaseous and sometimes have begun to condense into a liquid. Most of the time, the initial reactions connecting monomers and oligomers require high pressure and/or high temperature because the proximity of gaseous molecules at normal pressure and temperature is not conducive to the frequent or rapid occurrence of reactions. Catalysts can sometimes be used at this stage, too. Once a fairly decent sized molecule has been formed as described, the polymer may continue to grow in one of several ways. In the natural world polymers like proteins are generally built by continuing to add one monomer at a time. Most high school biology text books will have a good description of how this happens. In the case of polyethylene terephthalate, monomers are joined using a condensation polymerization reaction when ethylene glycol is combined with terephthalic acid, producing PET and water. All synthetic polymers are made using one or more type of polymerization.
Classroom activity: making a polymer
I will have the students make a polymer, but I am not sure if we will be able to make an actual polymer because of chemical/pregnancy safety concerns. Failing the ability to make an actual polymer, or possibly in addition to or in preparation for making the polymer, the students will make a model of a polymer, possibly even PET. Regardless of how the polymer is actually made, I want the students to see the waste that is created when plastics are made.
Chemistry in a bottle
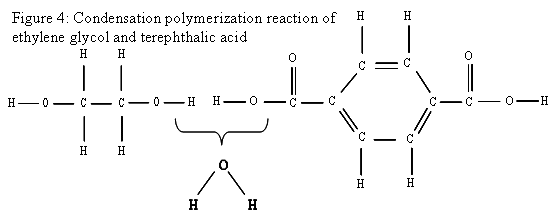
The life of a water bottle begins with the chemistry involved in making the plastic. Most water bottles, of the clear plastic type frequently sold in individual portions in the stores, are made primarily from polyethylene terephthalate, or PET (sometimes PETE). PET is a synthetic polymer made of repeating units of C 1 0H 8O 4. Figure 3 shows a diagram of the PET monomer. PET is generally made by the condensation polymerization ethylene glycol and either terephthalic acid or dimethyl terephthalate, creating a byproduct of either water or methane. Figure 4 shows where the water comes from in the condensation reaction between ethylene glycol and terephthalic acid. The diagrams shown in this section are intended for use both those who have a basic understanding of chemical equations. My students will have done some work with simple chemical equations by this point in the year, but they will probably require a bit more help to understand this section of the unit.
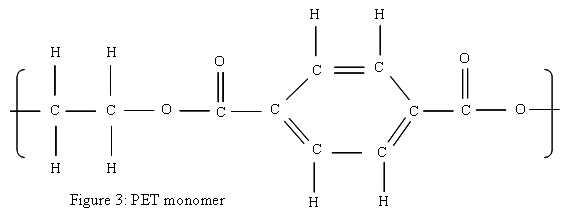
PET can be fairly rigid, which is inconvenient for most of its applications, so a plasticizer, di(2-ethylhexyl) phthalate or DEHP, among others is used to make it a bit more flexible. 8 Polymers, particularly long chains of polymers with some odd shapes sticking off them, get tangled easily. Think about a how easily a collection of computer cables can get tangled, and you will have some idea of what polymer chains can do. Plasticizers, as stated earlier, help the polymer chains to slide past each other and keep from tangling. The ease with which polymer chains slide past each other determines the final plastic's flexibility.
Class Activity: CAPT lab on physical properties of various plastics
This activity is one I am required to give some time before my students take their standardized test for the state in 10 th grade. The state asks questions on the standardized test about experimentation based on the series of experiments that the students do in the year and a half prior to the test. Since I have to teach the lab anyway and it seems to fit into this section of the unit, it seems like teaching it here is a good idea. The experiment may be found online at the link that is included in the resources section at the end of this unit. 9 In this experiment, the students are asked to examine several different types of plastics for tensile strength, puncture resistance, or abrasion resistance. Of the three, the first two are generally easiest to measure; however, with some creativity, the third can be measured as well. The teacher's version of this experiment may be helpful for those who have not done this experiment before.

Comments: