Background
The Structure of Matter
Atoms are the "building blocks" of all matter. Matter is anything that has mass and takes up space. As the sizes of various substances begin to move towards the nano-scale, their properties can become unique and dramatically different from the same substances in bulk form. To understand the affect size has on matter one must understand the structure of an atom.
Atoms are made of three smaller subatomic particles. Two subatomic particles are in the nucleus of an atom. The nucleus comprises the majority of the atom and is centrally located in an atom. The largest particles in the nucleus are protons. Protons have a positive electrical charge and the largest mass of the three subatomic particles. Neutrons are slightly smaller than the protons in the nucleus. Neutrons are neutral because they have no charge. Electrons are the last type subatomic particle. They possess a negative electrical charge, are very light and small in mass. Electrons are in constant motion and orbit around the nucleus at rapid speeds. Every element has a set number of protons, thus the protons determine each specific element on the periodic table. Electrons determine properties of materials such as conductivity, magnetism, and reactivity.
Forces and Matter
In nature, four forces affect atoms, gravitational forces, electromagnetic forces, strong nuclear forces, and weak forces. "Strong nuclear" forces hold protons and neutrons in the nucleus of an atom. This force is limited in range and has little influence beyond the range of the nucleus. Gravitational force is a function of mass and distance and is weak between nanosized particles. 6 Gravitational forces are negligible at the nanoscale because the mass of nanoscale objects is very small. Electromagnetic force is a function of charge and distance it is not affected by mass, so it can be very strong even when we have nanosized particles. 7 Electromagnetic forces occur between protons and electrons. The closer these charged particles are to each other the stronger the electromagnetic attraction. As the distance between these two charged particles increases, the electromagnetic attraction between them weakens. The forces that act on atoms can be attractive (unlike charges attract each other) or they can be repulsive (like charges repel each other). Electromagnetic forces act between the positively charged protons and negatively charged electrons. Electromagnetic forces help give an atom its shape and size. The size and shape of an atom depends upon the forces that attract electrons towards the nucleus and the repulsive forces that cause electrons to repel each other. An atom's ability to form chemical bonds depends upon the electromagnetic forces acting on the electrons found on the outermost energy level of an atom. When atoms do not have a complete outer energy level of electrons, they will transfer or share electrons with other atoms to become more stable. When atoms form chemical bonds with other atoms, they can form stable molecules.
Molecular Structure and Mechanical Properties of Matter
The atom carbon is a good example of how structural arrangements of atoms affect the properties of the resulting material. Carbon atoms have the ability to bond with many types of atoms, by means of covalent bonding (sharing of electrons to form chemical bonds). Carbon can form covalent bonds with four other atoms at the same time.
One common material formed when carbon combines with other carbon atoms is graphite. Graphite forms into sheets that are one carbon atom thick. These sheets can be large, with hydrogen atoms on their edge. The bonding that occurs between the sheets is due to weak forces called van der Waals' force. These weak bonds allow the graphite sheets to slide over each other. Therefore, when a pencil containing graphite moves across a piece of paper it leaves a trail of graphite. This property is the reason graphite is suitable as pencil lead and lubricants.
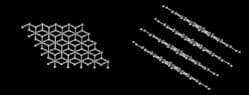
(Graphite)
Diamonds also form when carbon atoms bond together. However, when diamonds form the atoms arrange in neat stacks to form a three dimensional lattice type structure. In these three dimensional lattices, carbon atoms form bonds with each atoms immediately surrounding it in the lattice. A diamond's three-dimensional structural arrangement makes it stable and hard. Diamonds are the hardest known substance in nature.
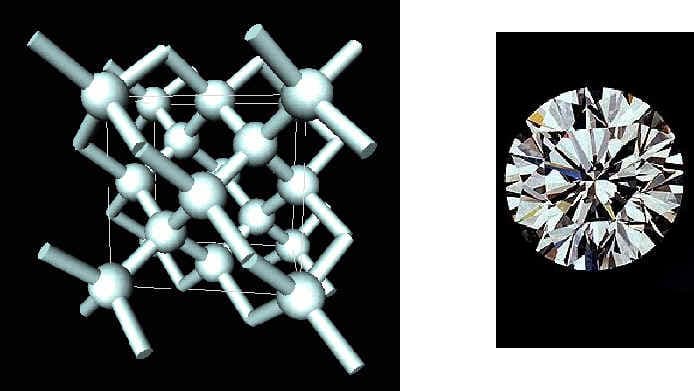
(Diamond)
When carbon atoms bond together at the nanoscale, they can form different structures, which are spherical or cylindrical in shape. Fullerenes, better known as "buckyballs," contain 60 carbon atoms arranged into a spherical lattice containing 12 hexagons and 30 pentagons. These tiny spheres are strong and are good conductors of electricity.
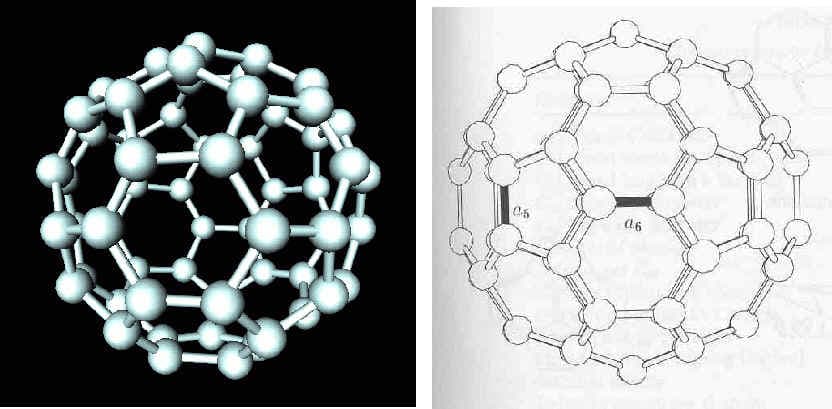
(Fullerene "Buckyball")
Carbon also forms structures called nanotubes. Nanotubes are tiny structures that have a chicken-wire type appearance when rolled up at different angles forming open or closed ended cylinders. These small structures can form into single walled cylinders or multi-walled structures. Nanotubes have great tensile strength because of their interlocking "carbon-to-carbon" covalent bonds, which form a large molecule. 8 Nanotubes are 100 times stronger than piece of steel that is the same diameter. They have the ability to resist bending. These structures are light and have a density that is about one-fourth the density of steel. They can conduct heat and cold, and have a high thermal conductivity.

(Nanotubes)
Size and Scale
What is size? The definition for size is based on the context in which it is used. Size can be the physical magnitude of something (how big it is). 9 Because differences in size are often apparent, we do not give it a lot of thought. Size governs the boundaries for all living and nonliving matter. If we were to compare the mass of a bee to a blue whale we find the bee has a mass of 100 mg or 10 -1g and the blue whale has a mass of 100 tons or 10 8g. However, do we really understand how size causes each animal to function differently and exhibit different properties in order for each animal to survive?
To define the size of various substances we must use some form of measurement or a tool of measurement. We use these tools to tell us how much or what amount of a given material exist. We use different tools to measure different materials. If we need to know the length of a solid, we use a ruler. To determine the volume of a liquid we may use a measuring cup or a graduated cylinder. Both of these tools use a reference point to help us determine the measure of the substances being observed. If we use some standard unit of measure to determine size, we are determining a quantitative or absolute size. 10 Reference points also help us make measurements. Reference points involve quantitative measurements. Quantitative measurements are absolute. If the disturbance is negligible, the object is large in an absolute sense; a nonnegligible disturbance means an object is small. Absolute size does not involve comparisons of one object to another.
Relative size is determined by comparing one object to another object; an object is large or small in comparison to another object. A relative measurement may involve comparing a rock to the palm of your hand. If the rock fits inside the hand, it would be considered small. If your hand could rest upon the rock, it would be considered large. Many students can make measurements that involve relative size; however, they may lack accuracy in making absolute or quantitative measurements.
Scale can be defined as an ordered reference standard such as a scale of 1 to 10. 11 Scale can also be considered the ratio of two measurements or the size of an object as compared to a model or representation of the object. 12 When we discuss the microscopic world, we examine changes in the scale of an object. Objects at the micro-scale can range in size from .1 µm to 100 µm or in regards to powers of ten from 10 2 nm to 10 5nm. The nanoscale, which is the scale below the micro scale, exists in the range of 1 nm to 100 nm or range from 10 -9 to 10 -7 meters. To be considered an object at the nanoscale at least one dimension of the object must fit within the nano-scale range (this is, one dimension must be less than 100nm). A DNA molecule is a good example of an object with one dimension in the nanoscale range. Although a single DNA molecule can be up to 5cm long it is only 2nm wide. The following website provides an excellent demonstration of how objects change in size as scales decrease: http://learn.genetics.utah.edu/content/begin/cells/scale/
Scientific notation is useful for describing objects at the nano-scale; scientific notation allows us to relate the size and scale of objects to well known metric units without using long strings of zeros as placeholders. To use scientific notation, one must understand that negative powers of ten are the reciprocal of the powers of ten. For example, if we stated an object was 10 -3 m, it means the object is 1/1000 of a meter. When working with substances smaller than one and greater than one the powers of ten become useful. Addressing objects based on varying scales helps one conceptualize how large or small an object really is. Use of scientific notation and metric units in science can help clarify size as it relates to scale.
Size and Strength
Strength is the amount of load an object can sustain before it breaks. 13 Strength is a mechanical property that is related to the physical structure of a material. 14 As an object gets smaller its relative strength increases, because of the number of imperfections an object has decrease as the size of the material decreases. The larger an object is the number of imperfections it has can increase. The more imperfections an object has the more weak spots it may have. Very small objects tend to have insensitivity to imperfections and are therefore stronger. Carbon nanotubes are very tiny and very strong. The size of these very tiny structures accounts for their increased strength.
Movies often depict gigantic monsters that have extraordinary strength. The gigantic beast is often depicted as a fast moving building wielding creatures. In reality, they would not be able to overcome the affects of size. In truth, King Kong's strength would be diminished because of his size. First, he would not be as active as portrayed because of his size. Second, the strength of a bone is proportional to its cross-sectional area. This means King Kong's bone structure could only support a maximum amount of mechanical force before it would break. The actual load a bone can withstand is proportional to the mass of the object. The hero in "The Incredible Shrinking Man was about an inch tall. In the movie, he was depicted as struggling to lift a needle. In reality, he would have been able to wield the needle around without any problem. His muscle strength would have increased 70 fold. The ratio of his ability to generate force is based on his body mass and would be 1/length; making him proportionally stronger. Smaller animals are proportionally stronger because the forces their muscles produce are proportional to the cross-sectional area. Their weight is proportional to its volume.
Surface Area and Size
Surface area and size change at disproportional rates when one dimension's length at a given scale changes. 15 The surface areas of nanoparticles are responsible for some properties at the nanoscale. As size decreases the surface area increases. For example, a packet of sugar, which has many small particles of sugar, would dissolve in a solution faster than a sugar cube, or large particle of sugar, because the packet of sugar particles has a greater surface area than the cube of sugar.
Surface area causes changes in the reaction time of a substance. The greater the surface to volume ration is as it relates to a reacting substance the faster the reaction time. The amount of exposed surface area increases drastically at the nanoscale level, which is the reason the reaction times for chemical reactions increase. Substances at the nanoscale level have a greater surface-to-volume ratio, which causes them to react very quickly. Small particles have a greater percentage of atoms on their surface, which accounts for the increased surface to volume ratio.
Size Dependent Properties
Properties are the characteristics that determine how a substance behaves, functions, or appear. Properties are generally measured by looking at large aggregations of atoms or molecules (~ 10 23). 16 Some properties are size dependent: the size or surface area of the particles determines the functionality, behavior, and appearance of the material. Size dependent properties can be categorized as size-dominated or surface-dominated.
Electrical properties can change at the nanoscale. Some materials that are conductors in bulk form may become semiconductors or poor conductors at the nanoscale. Some materials that were semiconductors may become conductors or superconductors. The confinement of electrons results in the electrical properties that occur at the nanoscale.
Optical properties are also size dependent. Electrons cannot move about as freely at the nanoscale and become restricted. The confinement of the electrons causes them to react to light differently. Gold for example will appear gold at the macro scale in bulk form. However when it occurs as nanosized particles its color is red. Nanosized zinc oxide particles will not scatter visible light, which causes sun block to appear transparent. Large zinc oxide particles used for sun block scatter visible light and appear white. Quantum dots change in their optical appearance as the size of the particles decrease creating different colors.
The second category of surface dominated properties involves properties controlled by their surface area. Melting point, rate of reaction, capillary action, and adhesion are properties that are controlled by their surface area. Gold provides an example of how melting points of a material can change with size. At the macro scale, gold has a melting point of 1064 º C. As its particle size decreases to the 100 nm to 10 nm diameter its melting temperatures drops about 100 ºC. As the size reduces to about 2 nm the melting point decreases to about half of the melting point at the macro scales level. Gold will no longer conduct electricity when it becomes less than 10nm.

Comments: