Background Information
What Is a Battery?
Simply put, a battery is a device used to store electrical charge as chemical energy. There are many different formulations available today, but they can be separated into two main divisions, non-rechargeable or primary cells and rechargeable or secondary cells. Non-rechargeable batteries will discharge energy through one discharge cycle, at which time all of the chemical components will have been transformed into new compounds in an irreversible process. Conversely, the chemical reactions in rechargeable batteries can be reversed when an external power source is applied. 1 Figure 1 below shows the primary components, as well as serving as a comparison between the two types.
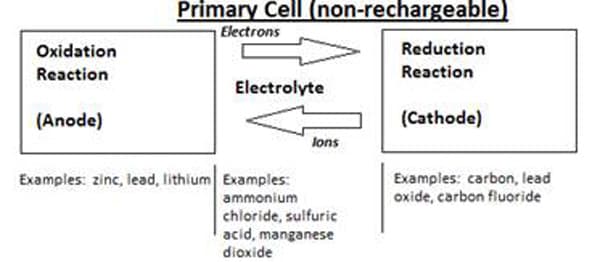
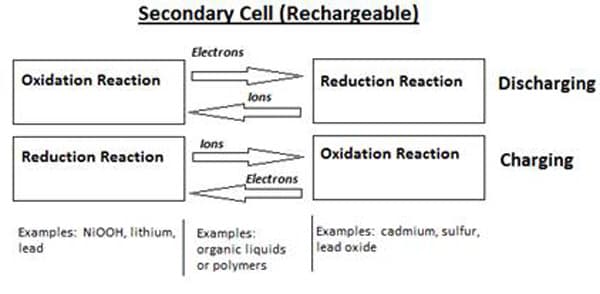
Figure 1
There are countless formulations for each type of battery, but a sample chemical reaction is shown below for a lead acid battery:
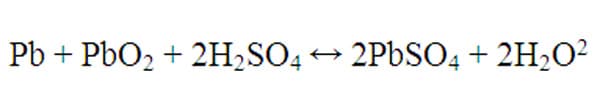
Note that the double headed arrow indicates that the reaction can proceed in both directions, indicating that this is a reaction that could occur in a rechargeable battery or secondary cell. Electrons would be freed when the reaction proceeds in the forwards direction, and adding electricity would cause the reaction to proceed in the reverse direction, thereby charging the battery.
Just as there are several formulations and chemical reactions used in today's batteries, there are also many different configurations. A generic diagram is shown below in Figure 2.
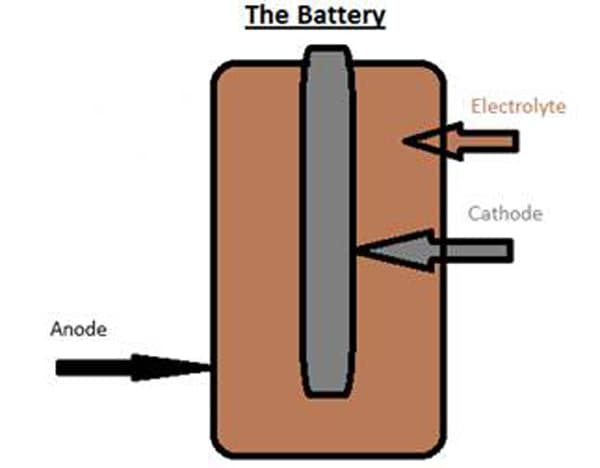
Figure 2: Shows a simple battery. The anode, where oxidation takes place, is shown in black, while the cathode, where reduction occurs, is shown in grey. The electrolyte is shown in brown.
A Brief History of the Battery
Given the extent to which electrical devices permeate everyday life, it is difficult to imagine a time "before" electricity. In fact, that is almost the case. While the dominance of electricity in society has occurred only over the past two centuries, electricity has been a source of wonder. As long ago as 1600 BC, amber, fossilized tree sap, was prized by the Greeks. They called it, "elektron" which came from the Greek word for gold due to its color. However, one of its most intriguing properties was that when rubbed, it would attract other objects. 2 Of course, we now know this phenomenon as static electricity. Since the amber was storing the electrical charge built up by rubbing it, Henry Schlesinger asserts that amber was, in fact, the first battery. 3
It was not until 1600 AD, when British physician, William Gilbert, published his book De Magnete, that this attraction was understood to be caused by some movement of particles inside the amber, rather than due to a transfer of heat caused by the rubbing or from magnetic properties. 3 In 1906, J. J. Thomson received the Nobel Prize for discovering the negatively charged subatomic particle that came to officially be known as the electron in 1914. 3
The first machine to hold a charge was the von Guericke generator, designed by Otto von Guericke in the mid 1600s. 3 This allowed both scientists and curious citizens to experiment with electrostatic charge. In 1706, Francis Hauksbee the Elder created a better device for storing electrostatic charge that enabled popular demonstrations. 3 One such gentleman, Charles-Fran?ois de Cisternay Du Fay, discovered that there were two charges, positive and negative. 3 By 1745, Ewald Jürgen von Kleist had used a device similar to von Guericke's generator to cause a glass filled with alcohol or mercury to glow. 3 This inspired others, such as Pieter van Musschenbroek and Andreas Cunaeus to create a device known as the Leyden Jar, "the first true electrical storage device." 3 This condenser/capacitor was replicated and used by other scientists as a source of electricity for their own experiments. 3 In an attempt to improve the jar, Daniel Gralath connected several jars in parallel and called it an "electrical battery." 3 However, it was Benjamin Franklin, shortly before performing his famous lightning experiment, who began to dissect the components of the Leyden Jar and improve upon them. 3 He, and others such as Thomas-Fran?ois D'Alibard, would later use the Leyden Jar as part of that famed experiment. 3
By 1800, Alessandro Volta published a work that described how to construct a battery, and he also created the voltaic pile. 3 Because, the pile used chemical energy, rather than needing a charge like a Leyden Jar, and could sustain the electrical impulse for longer periods of time, this was a major advancement. 3 However, he did not provide much, if any, explanation of what created the charge. 3
Shortly after this, Humphry Davy began to experiment with altering the materials used inside batteries, including the use of alkaline substances. Davy was also the first person to isolate lithium, which is the component of many batteries today. 3 Around this time, Antoine-Laurent Lavoisier had proposed that chemical and electrical affinity were the same. Davy demonstrated this through the synthesis and decomposition of water, concluding that electrical charges were holding the water molecules together. 3
Other scientists were also experimenting with using different materials, and William Cruickshank incorporated copper and zinc plates surrounded by acid, into a wooden box, creating the first battery that was mass produced. 3 His battery and others, however, had the annoying characteristic of accumulating hydrogen bubbles on their copper plate, necessitating that the plate be removed and cleaned. 3 Then, in 1836, John F. Daniell, created a battery that contained a porous vessel that separated copper sulfate and dilute sulfuric acid, which prevented build-up and allowed for continuous use. 3
Around this time, Faraday and a scientist named William Whewell began to use the term anode to describe the negative electrode, or the one that gives up the electron, and cathode to describe the positive electrode. 3 Meanwhile, Joseph Henry began experimenting with the use of multiple batteries and was the first person to create a parallel circuit, which resulted in greater amperage. By contrast, when he connected the batteries in series, the voltage increased. 3 Amperage and voltage have been described through the analogy of water in a hose, where volts are compared to the water pressure and amps are analogous to the volume of water in the pipe. 3 While working with electromagnetism, Henry also generated ideas that would later be used to design the telegraph. 3
In the ensuing years, batteries powered objects from boats to telegraphs and the Transatlantic Cable to doorbells and stock tickers. 3 Yet everyone was still working with batteries containing dilute acid. Then in 1866, Georges Leclanché created the a battery that utilized ammonium chloride, or sal ammoniac as its electrolyte, manganese dioxide as its cathode and zinc as its anode. 3 This was a huge advantage, since it was less corrosive and could be replaced more easily by the average user. However, because it was suitable for only intermittent use, it worked best in applications such as doorbells. 3
Another scientist, Gaston Planté, was active in battery research at this time as well. By 1889, he had developed the first rechargeable battery, the lead-acid battery. 3 Among other things, these were used for electric lighting. The well to do could rent sets of batteries and strings of electric lights, along with an electrician to maintain them, for a contracted amount of time during special events. 3 Alternately, a party host could also utilize the services of the Electric Girl Light Company, who would
"furnish a beautiful girl of fifty or one hundred candle power, who will be on duty from dusk till midnight-or as much later as may be desired…This girl will remain seated in the hall until someone rings the front doorbell. She will then turn on her electric light, open the door, and admit the visitor and light him into the reception room. If, however, any householder should desire to keep the electric girl constantly burning and to employ another servant to answer the bell, there can be no doubt that the electric girl, posing in a picturesque attitude, will add much to the decoration of the house."4
From theatrical costumes to electric irons, fans, sewing machines, automobiles, the flashlight, the toaster, the electric handshake buzzer used in practical jokes today, electric pens, and the phonograph, the battery was making its way into everyday life. 3 At the 1893 World's Columbian Exposition in Chicago, it also powered moving sidewalks, the Electric Building, and small boats. 3 Furthermore, the battery enabled Elisha Gray and Alexander Graham Bell to invent the telephone, but it had its downside as well. Excited by the promise of this magical power, some began to apply it to medicine, leading to applications that ranged from quackery to dangerous. 3
One major drawback of the battery during this time, however, was that it still required much maintenance. Felix Lalande and George Chaperson developed a battery, now referred to as the Lalande battery that placed copper and zinc electrodes into an alkaline solution housed in a ceramic container. 3 The only maintenance required was to periodically change the electrolyte, making the job of those who maintained the telegraph lines much simpler. Lalande also created a version which housed the components inside a sealed, porcelain container, making it suitable for use in the home. 3
Meanwhile, Carl Gassner was inventing the first dry cell. 3 Dry cells were maintenance free and are the batteries still used today in most simple consumer electronics. It was both applied to existing technology, such as telegraphs, as well as allowed the creation of a wide array of novelty items. 3 Companies such as Ever Ready and Energizer and the precursors to Rayovac and Duracell were born. 3
By the turn of the 20 th century, batteries were powering the wireless telegraph, which was brought to the public by Guglielmo Marconi. Radio soon followed, with applications during the first World War and popularity both commercially and in the hands of hobbyists. 3 Perhaps the most major development, however, came between 1917 and 1919, when the National Bureau of Standards met with representatives from industry to develop a set of specifications for the standardization of batteries. 3 In doing so, they not only noted size and voltage, but also gave the batteries names. 3 This was of huge benefit to consumers. Although the power grid was advancing and electricity was becoming increasingly commonplace for applications such as lighting and radios in urban areas, rural areas still relied exclusively upon batteries. 3 Samuel Ruben even merged the grid and the battery through the invention of a device called the "trickle charger," which allowed batteries to remain charged by connecting them to a household outlet. 3
World War II saw increased use of the battery for countless portable devices. It also led to the creation of a battery called the proximity fuse, which could be placed inside a shell and did not began to discharge until the shell was fired. 3 Other great advancements were made during this time by companies such as Motorola in terms of decreasing the size and weight of batteries used for communications. 3 However, batteries were still very sensitive to the environments in which they operated. For example, the heat and humidity of combat in tropical environments caused batteries to run down too quickly. 3 This was remedied by Samuel Ruben, who made the first new chemical advance in batteries in a century, when he created the mercury cell. 3 The P. R. Mallory Company, which later became Duracell, packaged them in steel and churned them out for the troops. 3 They would later be used in the exploration of space. 3
Although other substitutions were also made, such as including cadmium in place of mercury, the limitations of batteries were eventually reached when appliances, such as radios, required more than a few vacuum tubes for operation. 3 Faraday had previously described the First Law of Electrolysis, which states that "in order to double the output of any battery, the amount of material in that battery must be doubled." 5 Nonetheless, new applications, such as the battery powered wristwatch, continued to appear. 3 Then in the middle of the 20 th century, John Bardeen and Walter Brattain of Bell Labs invented the transistor, eliminating the need for vacuum tubes and enabling batteries to once again play a prominent role in electronics. 3 The common dry cell also made advancements during this time period, when Lewis Frederick Urry created an alkaline battery which utilized powdered zinc, thereby greatly increasing the surface area for chemical reaction to take place and giving it a much longer lifespan. 3
Other advances in electronics, such as the development of the integrated circuit or IC, which decreased the size and increased the portability of electronics, also propelled the battery forward in terms of its utility for everyday life. 3 As consumer electronics continued to advance, so did the batteries used to power them. The end of the 20 th century saw the creation of the "jelly roll" battery, in which the battery components were rolled together to increase the surface area of the components. 3 Rechargeable batteries also increased in use so that alkaline, nickel cadmium, NiMH, and lithium batteries all increased in prevalence. 3
Yet Faraday's Law continues to plague us today. Alkaline batteries seem suited only for use in applications requiring relatively low voltage, while lithium-ion batteries have the most use, and NiMH batteries are somewhere in between. 3 Due to their toxicity and precise specifications needed to prolong battery life, nickel cadmium batteries are decreasing in terms of use. 3
Meanwhile, lithium-ion batteries are at the forefront of today's battery use. Lithium was first discovered as lithium aluminum silicate or petalite in Sweden in 1800. 3 Ten years later it was classified by Johan Arfwedson and named lithos because he believed it to be alkaline. 3 In the 1970s, Exxon and Eveready, attracted by the "high energy density" that seemed to defy Faraday's Law, 3 began to study its application to batteries. Due to safety concerns, however, they abandoned their study. NASA found limited applications in the decade that followed, but in the 1990s Sony and the Asahi Chemical Company brought the lithium-ion battery to the consumer market in full force. 3 Other Asian companies soon followed, and today they are a mainstay of consumer electronics. 3
For the most part, however, lithium-ion batteries do not represent a significant shift in how batteries operate. Furthermore, for the last 200 years, objects have been created to work with the batteries that exist, so there has been little incentive for major advancements in battery technology. 3 Such an impetus may be close, however, due to the need to develop storage capacity for renewable energy. Although there is some interest in the advancement of other batteries such as the lead-acid battery, 6 most of the advances in battery technology are designed to work with a battery that utilizes lithium as an electrode. These include batteries in which sugar or alcohol is utilized as an electrolyte and even one in which urine is the electrolyte. 7 Another bio battery incorporates the bacteriophage M13 to produce the peptides that form single-walled carbon nanotubes for the electrode. 8 For others, the emphasis is on design in terms of consumer wants, such as batteries that will flex or bend. 9
Unfortunately, although some advancements have been made, lithium batteries still pose a safety concern, so this is also an area in which progress can be made. One solution incorporates polyethylene microcapsules at the anode. Because lithium-ion batteries are known to overheat, causing fires, the capsules are designed to melt at increased temperature, stopping the movement of ions and thereby putting out the fire. 10 The self-healing polymer could also fill cracks caused by mechanical stress and prolong battery life. 1 1
Development is also needed in terms of finding renewable sources of battery components and maximizing stored energy. 11 Such advancements could make them more competitive for storing energy produced via solar, wind, nuclear and geothermal resources. 12 Of course, we must also remain cognizant of the methods by which batteries are disposed. Concern has been raised most recently regarding the potential for zinc pollution due to litter 13 and incineration. 14
Despite current limits of supply and concern regarding disposal, batteries may hold the key to our future energy needs. In addition, there is research on ultracapacitors and fuel cells that is promising. 15 As we seek to decrease greenhouse gas emissions and combat climate change, technologies such as solar and wind power become increasingly desirable means of meeting the needs of a power hungry, technological society. Unfortunately, the electricity produced via these means is intermittent due to the fact that the sun does not shine at night and wind power is unpredictable. Batteries have the potential to capture excess energy produced during the daytime or high winds for later use. Furthermore, other alternative energies, such as nuclear and geothermal energy can also benefit. 13 Because nuclear reactors supply a constant energy source while consumer use reflects times of peak consumption followed by periods of lower demand, the development of batteries and similar devices to store energy could minimize waste and enable us to meet the need during times of high demand more efficiently. Meanwhile resources such as geothermal and tidal power are limited in terms of location, so a device such as a battery would enable power from these sources to be transported to areas where those resources were previously not an option. These ideas will form the basis for the culminating activity for my unit.

Comments: