Appendix C
Students will engage in a laboratory activity to build a battery. In Part A, students will examine a "potato clock." This is made by inserting pieces of zinc and copper into a potato and connecting them to wires that are attached to an LCD clock. A diagram is shown below in Figure 3.
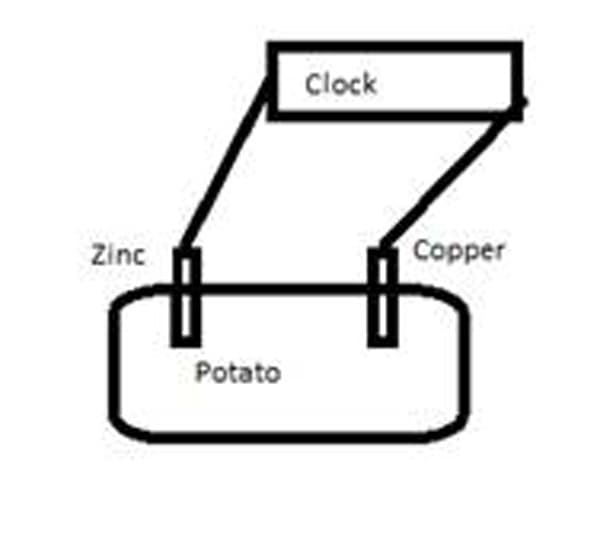
Figure 3
Students will answer questions about the set up and then be asked to create a battery using an item other than a potato. Student directions and questions aimed at understanding experimental design are included below.
In Part B, students will follow a procedure from our chemistry text 17 to create a battery using copper, zinc and sodium nitrate solutions. A picture is shown below in Figure 4 that demonstrates much of the setup, with the exception of a salt bridge, which can be made by filling a u tube with electrolyte and plugging the ends with glass wool or by soaking a paper towel in the electrolyte solution and placing each end into the beakers containing the electrodes below the solution level. The students will use this battery to light a light bulb.

Figure 4
Battery Lab Part A
1) Examine the clock. How does it get its power? Make a sketch of what you see and label the role of each object (potatoes, wires, etc.)
2) What might be the effect of altering the set-up? What would happen if you used an orange, instead of a potato? What if you changed the size of the metal or wires?
3) Select one change that you would like to make. What we change is a variable. Is this an independent variable or a dependent variable?
4) Predict what will happen when you make this change. Your prediction is a hypothesis. Please state it in "If…then…because" format.
5) Draw a diagram of your new set-up. Show it to your teacher for approval.
6) Set up your new apparatus. What happens? Do the results support or disprove your hypothesis?
Battery Lab Part B
Follow the procedure for Part B: Making a Battery found on pages 309-311 of the Active Chemistry book. Your teacher will put the answer to step 4 on the board for you, and we will discuss it at the end of the lab.
**Extension (Extra Credit): What effect do you think varying the solutions contained in the two beakers would have?

Comments: