Background
Polymers
Polymers are very long chained molecules made up of repeating units called monomers. There may be up to 1,000 units for one polymer strand. Recognition that polymeric macromolecules make up many important natural materials was followed by the creation of synthetic analogs having a variety of properties. Applications of these materials as fibers, flexible films, adhesives, resistant paints and tough light solids are part of our everyday lives. 5 I will study the structure of various polymers and relate the variation in properties to the differences in structure.
A chemical polymer-forming reaction will cause one molecule to add on to another and so continue, until a very long strand has formed. Ethylene (ethene) is the monomer for the corresponding polymer called high density polyethylene (HDPE).
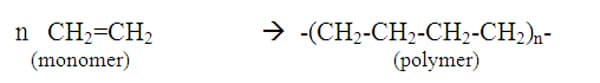
Unlike simpler pure compounds, most polymers are not composed of identical molecules. The HDPE molecule for example are all long carbon chains, but may vary by thousands of monomer units with a range of molecular masses from 2 x 10 5 to 3 x 10 6. 5 Some other addition polymers are our most common plastics (Table 1).
Table 1. 5
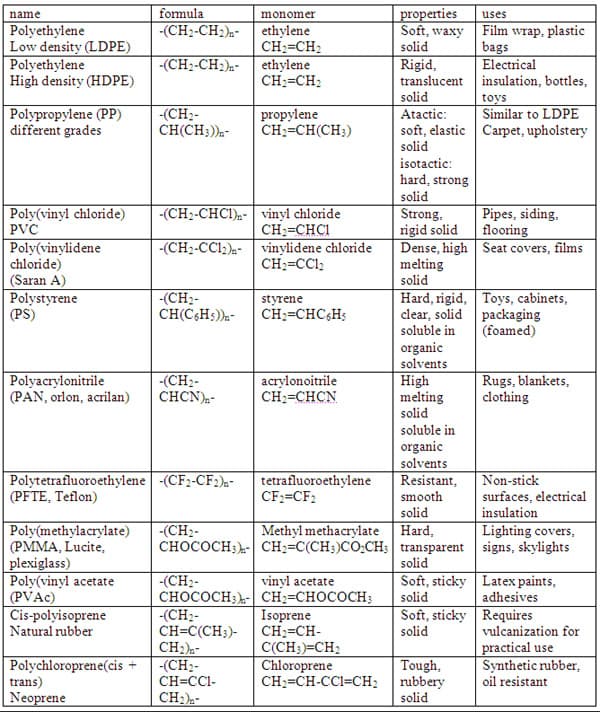
Properties of Macromolecules
A comparison of properties of polyethylene (both LDPE & HDPE) shows that even though they are comprised of the same monomer the properties are not the same. HDPE is a rigid translucent solid which softens on heating above 100 oC and can be fashioned into various forms including films. It is not easily stretched and deformed as is LDPE. Both HDPE and LDPE are insoluble in water. LDPE is a soft translucent solid which deforms badly above 75 oC. Films made from LDPE stretch easily and are commonly used for wrapping. To account for these differences, the nature of the aggregate macromolecular structure, or morphology, of each substance needs to be considered. Polymer molecules are very large and so they tend to pack together in a non-uniform fashion, with ordered crystalline regions mixed together in with disordered or amorphous domains. In some cases, the entire solid may be amorphous, composed entirely of tangled macromolecular chains. Crystallinity occurs when linear polymer chains are structurally oriented in a uniform three dimensional matrix. Increased crystallinity or order is associated with an increase in rigidity, tensile strength and opacity (due to light scattering). Amorphous polymers are usually less rigid, weaker and more easily deformed. They are often transparent. Three factors that influence the degree of crystallinity are: Chain length, chain branching, and inter-chain bonding. 5
The importance of the first two factors can explain the differences in properties between LPE and HDPE. HDPE is made up of very long unbranched hydrocarbon chains. These pack together easily in crystalline domains that alternate with amorphous segments, and the resulting material, while relatively strong and stiff, retains a degree of flexibility. In contrast, LDPE is composed of smaller and more highly branched chains which will not easily adopt crystalline structures. This material is, therefore, softer, weaker and less dense and more easily deformed than HDPE. As a rule, mechanical properties such as ductility, tensile strength, and hardness rise and eventually level off with increasing chain length. 5
Inter-chain bonding is what vulcanization does to rubber to stiffen it and make it a useful material. Natural rubber is an amorphous polymer and so is soft and liable to lose all usefulness at high temperatures. The chains of rubber molecules become cross-linked by adding sulfur to the chains and sulfur to sulfur bonds hold the polymer chains together. The degree of crosslinking determines the hardness and strength. At 2-3% crosslinking, a useful soft rubber forms that no longer suffers from the stickiness and brittleness problems on heating and cooling. At 25-35 % crosslinking, a rigid hard rubber product is formed. 6
On heating and cooling most polymers undergo thermal transitions that are related to their morphology. These are defined as T m the melt transition which is the temperature at which crystalline domains lose their structure or melt. T m is an indication of the degree of crystallinity. T g is the temperature below which amorphous domains lose their structural mobility of the polymer chains and become rigid glasses. It is often interpreted as the temperature above which significant portions of polymer chains are able to slide past each other. The introduction of relatively large and stiff substituents (such as benzene rings) will interfere with this chain movement and so lead to a higher T g value. Polystyrene has benzene attached to the ethylene monomer and has a higher T g value than those of other polyethylenes. Elastomers are amorphous polymers that have the ability to stretch and then return to their original shape above T g. This property is important in applications such as gaskets and O-rings, so the development of synthetic elastomers that can function under harsh or demanding conditions remains a practical goal. At temperatures below T g , elastomers become rigid glassy solids and lose all elasticity. A tragic example of this caused the space shuttle Challenger accident. The heat- and chemical-resistant O-rings used to seal sections of the solid booster rockets had an unfortunately high T g value near 0 oC. The temperature was low that morning and below the T g allowing hot rocket gases to escape the seals. Thermoplastic polymers may be shaped or pressed into molds above T g. Even after cooling, the hardened polymer will lose its shape if once again heated above T g. Whereas, thermosets are polymers which once formed cannot be reshaped by heating. 5
Another level of complexity
Symmetrical monomers such as ethylene and tetrafluoroethylene can join together in only one way. Monosubstituted monomers can join together in two organized ways or one random manner. The orientation of the substituted group (Z) is what varies and it can be above or below the plane defined by the carbon chain. One version has Z on one side of the plane – called isotactic. The other symmetric version has Z alternating from one side to the other of the chain in a regular manner – syndiotactic. The other option is to have Z randomly arranged about the chain – atactic. 6 Many common and useful polymers, such as polystyrene, polyacrylonitrile, polyvinyl chloride, and propylene are atactic as normally prepared, but catalysts may control the process and produce just one of any three forms. The properties of a given polymer will vary considerably with its tacticity. Atactic polypropylene is useless as a solid construction material and is used mainly as an adhesive. In contrast, isotactic polypropylene molecules will line up in straight chains and so have a much higher melting point and better crystalline properties. 5
How can different structures result for the same monomers? The formation of addition polymers must be initiated by some kind of radical - an atom or molecule lacking a complete electron shell and looking to form a bond by sharing an electron with another atom. Once the reaction starts and proceeds for some time, the reaction chamber will become crowded with many different free radicals which may combine with other monomers or with another free radical. This combination of two radical chains stops the continued building of a chain. This chain though may combine with another radical fragment and so branched molecules form. If the reactions proceed unchecked, a mixture of branch lengths and branching will occur. Catalysts can be used to control the level of branching and, hence, the properties. 5
The synthesis of macromolecules composed of more than one monomeric repeating unit has been explored as a means of fine tuning or increasing the properties of the resulting material. These are called copolymers. The molecules form blocks and different blocks are covalently bonded together. These can be combined in symmetric patterns, randomly or in blocks depending on the properties sought. One of the most commercially important block copolymer consists of blocks of polybutadiene sandwiched between two blocks of polystyrene. The polystyrene units aggregate into clusters linked together via polybutadiene chains. The clusters are stiff, like polystyrene itself; but the polybutadiene chains are flexible, and the material behaves like a rubbery elastomer. Unlike rubber, this polymer can be molded and is used to make the soles of sneakers. 6
Nylon is an example of a condensation polymer where an ester linkage joins the monomers. With these polymers, there is more opportunity for hydrogen bonding intermolecular attractions and, hence, tougher materials. Polyester, spandex and Kevlar are other examples of condensation polymers. 7
Natural versus Synthetic Polymers
The reactions to form synthetic polymers are difficult to control and often do not lead to the consistent structures needed to provide given properties. Natural polymers on the other hand are self-assembled, can be coded by DNA, and have a complexity of structure that the synthetic processes can never achieve. 1
The body creates a variety of different materials: skin which is elastic yet resistant to penetration by water and other fluids, nails which are much tougher and more brittle, and hair which is extremely difficult to break. The basis of these is proteins. Proteins are condensation polymers using a combination of twenty-two possible amino acids, where the acids used and the order of combination being determined by DNA. When the type of amino acid is varied and the order changed, then a very different protein is formed with a very different shape and properties. The proteins can be woven into strong fibers, cross-linked or entangled; they form the stiff matrix of horn and claw or elastic sheets. Their construction is encoded in our genes. 1
The most abundant structural protein in the human body, comprising about one quarter of our total protein mass is collagen. This is a relatively simple protein whose chainlike molecules contain mainly glycine and proline. Collagen shows the major difference between natural protein-based structural materials compared with most synthetic polymer-based plastics. Both are composed of long chains of molecules but, in the natural materials, the chains gather together in complex arrangements, forming thicker groups like ropes woven together, like string woven from thread. Each collagen molecular chain crimples up into a helix. Three of these twist around each other to form a rope-like microfibril. These microfibrils aggregate together in various ways. For example, they can gather together in a staggered arrangement to form thick strands called banded fibrils. These constitute the connective tissue between cells. They hold our flesh together. In the eye's cornea, the fibrils are packed close together in a very orderly pattern. These fibrils are too small to scatter light and so the material is virtually transparent. Collagen has a fibrous structure at the molecular and microscopic level but the body organizes them into other shapes depending on the need. 1
A spider silk is a fiber stronger than steel. This silk can be spun into almost invisible threads which can withstand the impact of flying insects. These impressive properties are a consequence of the way its protein chains are organized. In silk, the basic organized structural element is not a coil but a sheet. Neighboring chains sit side by side in aligned ranks and each chain is linked to those on either side via hydrogen bonding, which zip the chains together in beta sheets. The orderly, relatively rigid sheets can stack on top of one another to create tiny, three-dimensional protein crystallites. These crystallites are microscopic and become dispersed in the tangled, flexible protein matrix and form a copolymer. So, the silk has both strength and flexibility. 1
Silk fibers are insoluble in water- otherwise they would dissolve in rain or dew. The spider spins the threads from a solution of protein molecules in water, thereby achieving the remarkable feat of changing a soluble substance into an insoluble one. This change in solubility is a result of a change in the way that the chains are organized. The spider manufactures silk protein in its silk gland, at which point it is still soluble. This solution passes from the gland towards the spinneret. During this stage the silk solution loses water and becomes more concentrated. The proteins rather than being hydrogen bonded to the water now become cross-linked to each other. By the time the silk leaves the spinneret most of the water has been squeezed out and the proteins have formed beta sheets. The molecules in these crystalline regions are so closely packed together that it is difficult for the water molecules to penetrate, and so, the silk fibers are essentially solid and insoluble. 6
For the spider, its DNA contains the blueprint for making the polymer silk. Biotechnology is a means to use nature's controlled building of the molecule into sophisticated structures. To produce a fiber stronger than steel on a larger scale than a spider's web, the spider gene has been inserted into goats and the hope is to duplicate the extrusion process of the spinneret to make a silk from the goat's milk. The responsible gene can also be spliced into the DNA of host bacteria – Escherichia coli – and then the bacteria proceed to translate the synthetic genes into polypeptides. At the moment, these processes are too expensive to replace the commercial processes of the giant plastics industry. 1
Besides control and enhanced structure, natural polymers are biodegradable and do not leave their imprint on the environment. Plastics on the other hand have filled our landfills and even created toxic waste. Most of us separate out our plastics on trash day and assume that they will be recycled. Plastic recycling mainly comprises re-use but normally as lower grade plastic. Much recycled plastic is used to make shopping bags and at some point we should not need as many. In the life of a plastic, there becomes a time where the recycled plastic no longer has a use and so once again will need to be added to a landfill or burned. We are slowly learning that when we design new materials that we need to look at what happens to them after their useful lifetime. Re-use will only go so far and better planning would have the molecules broken down to safely enter the environment. See appendix 2 for the principles of green Chemistry.
Carbon and Nanotubes
Technology is such that we can build molecules atom by atom and isolate these molecules or very few clusters of molecules. It seems appropriate to look at some examples of this, as this is the new materials science. Elemental carbon is an example of how structure explains properties. Diamond is one of the hardest materials known. This is due to all carbon atoms being bonded to four other carbons and forming a network with a tetrahedral shape around each carbon. Since each atom is bonded in this highly symmetrical shaped pattern, the resulting structure is very strong. In graphite, the carbon atoms, though, are arranged in sheets of co-joined hexagons where each carbon atom is bonded to three other carbons – two single bonds and one double bond. These sheets then experience weak London intermolecular forces between them. The layers can easily slide past one another and so graphite flakes easily. Buckyballs or Fullerenes are nanometer sized and one of these, C 6 0, contains sixty carbon atoms arranged like the vertices for the sections of a soccer ball. 8
Graphene is a single layer of graphite that displays incredible properties of electron conductivity, strength and stiffness. Three million sheets of graphene are equivalent to 1mm. Graphene's electrons move one hundred to one thousand times faster than those of silicon. Therefore, there is potential to replace silicon and provide even smaller computer chips with even faster computing. 9 The ability to conduct electrons means that it would be very useful for applications where quick energy surges are needed, such as in electric cars or stabilizing energy grids. Graphene has a breaking strength two hundred times greater than that of steel. A sheet of graphene, as thick as a piece of cellophane, would support the weight of a car. If paper was as stiff as graphene, you could hold a one hundred yard long sheet of it at one end without it breaking or bending. 1 0 Small flakes of graphene can be mixed with other materials to form composite materials which can be used to build stronger and lighter products. Sheets of graphene can be folded in several ways to form tubes which are used in many applications. Different folds give different properties and the challenge of nano-science is finding a way to control the type of tube produced. Nanotubes are used in flat screen televisions, high electrical conductivity applications, storing hydrogen for fuel cells, artificial muscles, sensors, transistors and the delivery of drugs. Nanotubes have been mixed with nylon to form very small components. The carbon nanotubes have excellent thermal conductivity properties that cause the material to cool slowly and evenly, allowing for better molding characteristics of the nano-composite. The slowing of the process allows the composite molecules to fill the tiny micron-sized mold. As of now, graphene and nanotubes cannot be produced either cheaply or on a large scale, but the potential is there. 1 1
Professor Alexander Star at the University of Pittsburgh is conducting research using nanotubes in novel applications such as the production of chemical and biological sensors, energy conversion devices (fuel cells) and nanocapsules for drug delivery. As one-dimensional structures electrons are confined to the exterior of the nanotube, making them extremely sensitive to perturbations in the local environment. Carbon nanotubes decorated with metal nanoparticles exhibit unique selectivity for absorbing gas molecules, with the potential for detecting nitrogen gas and warning asthma patients before an attack or detecting oxygen levels in mines. He has also developed nitrogen-doped nanocups which show good catalytic properties for the Oxygen Reduction Reaction, which makes them attractive substitutes for metals such as Pt or Ru in fuel cell cathodes. Dr Star's group has been able to form nanocapsules with the potential to be used as incredibly specific drug delivery systems. Currently, it is known that carbon nanotubes can cause negative effects such as inflammation, oxidative stress and cell death. His group has shown a natural biodegradation of single-walled carbon nanotubes through enzymatic catalysis. I have seen a presentation by Dr. Star and he is available to give a talk to my class about his interesting research. 1 2
Molecules Used in Demonstrations/Labs
Sodium polyacrylate can absorb almost eight hundred times its mass in water. It is used in diapers and to regulate water around plants in agriculture. Sodium polyacrylate is obtained by the co-polymerization of sodium acrylate and acrylic acid, in the presence of a cross-linking agent. The two monomers form a random copolymer, with sodium acrylate and acrylic acid repeating units randomly distributed within a typical polymer molecule. The cross-linking agent is a reactive molecule that can 'insert' itself into two or more growing polymer chains as the polymerization reaction continues. This serves to tie together several polymer chains into a large, three dimensional, polymer network. Extensive cross-linking makes the polymer insoluble in water and creates a membrane-like barrier on the surface of the polymer that allows water to flow inside. The presence of the –CO 2 - groups in the polymer structure means that there is also a high concentration of sodium ions within the polymer network. This creates a concentration imbalance or gradient when the polymer is added to water. Osmosis occurs and the water molecules diffuse across the membrane to equilibrate the sodium ion concentration inside and outside the polymer. Once inside the polymer network, the water molecules form hydrogen bonds with the ionic –CO 2 - groups, creating a thick viscous, translucent gel. The amount of water that can be absorbed by the polymer depends on the ratio of –CO 2 - and –CO 2H, the amount of or extent of cross-linking, and the concentration of sodium ions. When sodium ions are added to this gel, then the flow of water is reversed out of the polymer network and a slurry forms. 7
PolySnow T M is a highly cross-linked formulation of sodium polyacrylate. The extra cross-linking means that PolySnow absorbs less water than sodium polyacrylate. PolySnow also does not form a typical gel when it absorbs water. The powder absorbs water to form white particles that look like snow. 7
Sodium alginate
Sodium alginate is a natural polymer obtained from kelp and seaweed. The polymer is a typical component of the cell wall in brown algae, comprising up to 40% of the dry weight of large species such as giant kelp. Worldwide, about 16 million pounds of sodium alginate are produced per year for use in the food, textile, medical, and pharmaceutical companies. 7
Sodium alginate is a polysaccharide composed of thousands of oxidized sugar units joined together to form an ionic polymer. The repeating units are six-membered rings containing negatively charged –CO 2 - groups. An oxygen atom connects each ring. The presence of –CO 2 - side chains, as well as numerous –OH groups, make this polymer hydrophilic or 'water loving.' The polymer readily absorbs water and will swell up with contact with water to form a gel. The resulting gel is thick, viscous, and smooth. Sodium alginate is used as a thickening agent in many processed foods including ice-cream, yogurt, and artificial food snacks. The non-toxic food additive absorbs water, helps to emulsify oil and water components, and gives foods a smooth texture. 7 Replacing the sodium ions in sodium alginate with calcium ions gives an insoluble product, calcium alginate. Encapsulation of liquids or solids within a membrane is a convenient method to protect materials or allow their gradual release. Encapsulated or microencapsulated materials, such as pharmaceuticals, insecticides, toxic wastes, foods, inks, catalysts and bioartificial organs are common. Controlled pore size in or degradation of the membranes can result in timed release from minutes to months. The mild gelation of alginate solutions in the presence of calcium ions has allowed the entrapment of DNA, proteins, and cells in cross-linked matrices without loss of biological activity. 1 3

Comments: