Appendix D: Student Activities
Student Activity 1
Objective: Students will identify pollution.
Essential Question: What is pollution? What is not pollution?
Procedure:
1. Teacher will show the students a short powerpoint of pictures depicting pollution. Preferably, the pictures will come from surrounding area.
2. Students will work in cooperative groups of 3-4, and brainstorm their ideas to define pollution.
3. Class will discuss and brainstorm together the ideas to define pollution.
Closure: Class will create a definition for pollution.
Student Activity 2
Objective: Students will model a polymer.
Procedure:
1. Students will line up and link their arms to model how a polymer is linked.
2. Another way the students can model the polymers is to link paper clips.
3. Other polymer examples can be collected and displayed for observation, such as gum,
gummy worms, rubber bands, synthetic fabric, nylon stockings, pencil erasers, CD cases, and balloons.
Student Activity 3
Objective: Students will compare the unique chemical reaction of a polymer with a simple solution.
Essential Question: What is different between a polymer and a powdered drink mix?
Procedure:
Students will make a powdered drink mix in a glass, such as Kool-aid.
1. Students will mix 1 teaspoon Borax (sodium tetraborate) in 8 oz. of water. (Distilled water works slightly better than tap water.)
2. Students mix equal amounts of glue and water, mixing thoroughly, 8 oz. bottle with 8 oz. of water works well. (You can add a couple drops of food coloring if you want.)
3. Now slowly add the Borax solution to the glue solution, and knead with your hands, or put into Ziploc bags to mix.
4. Students will observe the characteristics of the silly putty ball.
5. Silly putty ball may be stored in a sealed Ziploc bag to retain its properties.
6. Students will compare the difference between the silly putty ball and the glue, water, and borax with the powdered drink mix and the water and powder.
7. Can the silly putty ball return to the original ingredients? Can the powdered drink return to the original ingredients?
8. The glue already has a vinyl polymer in it, but the water helps the vinyl molecule to untangle. Once the Borax is added, it creates the cross-linking of the molecules.
9. Other polymer examples can be collected, such as gum, gummy worms, rubber bands, synthetic fabric, nylon stockings, pencil erasers, CD cases, and balloons.
Closure: Students will see that the ingredients of polymers don't look or act like the resulting polymer. Polymers have rather unique characteristics.
Student Activity 4
Objective: Students will compare a model landfill with a liner, to a model landfill without a liner, note the differences in the water below and analyze the potential differences in an environment.
Procedure:
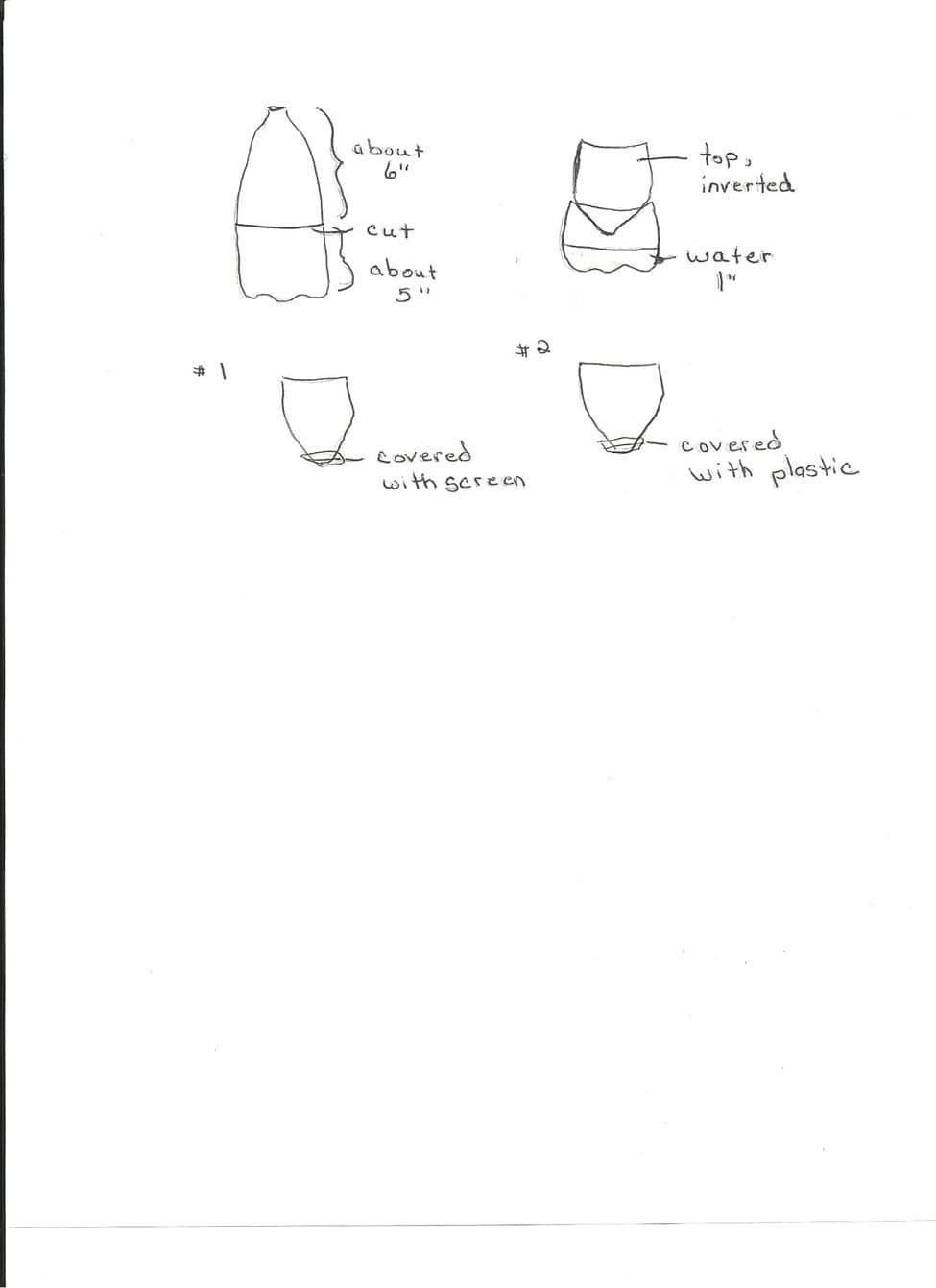
1. Students will use two, 2-liter soda bottles to construct model landfills. The teacher will cut the bottles into 2 pieces. The bottom piece should be about 5 inches high. The upper piece should be about 6 inches high. The upper piece will be inverted into the bottom piece. (See diagram A.) The first landfill model will have a piece of plastic covering the opening of the bottle, fastened with a rubber band. The other bottle will have a small piece of screen, fastened with a rubber band. On top of the plastic or screen a small layer of gravel should be added. On top of the gravel a layer of soil, about 3 inches should be added.
2. About 1 inch of water should be added to the bottom layer, representing ground water.
3. Next the students should simulate adding things to the landfill. They should add: "oil" in solution with water (red food coloring, water, and vegetable oil can be used).
4. Students will observe the water in the bottom, noticing any changes.
5. Questions:
What did you notice in the water of the landfill with the liner?
What did you notice in the water of the landfill without the liner? Why did this occur?
What do you think would happen in real life? Why? What do you think could happen to the environment? What might be some of the contaminants that could be dumped into a landfill? Which model would be best for the environment? Why?
Closure: Students will note that any contaminants added to the landfill without a plastic liner would eventually leach down into the ground and groundwater below it.
Diagram A:
Student Activity 5
Objective: Students will determine how well various substances decompose.
Procedure:
1. Students will set a deep dishpan or plastic container to create a model compost pile. It is best if the container has small drain holes in the bottom, so that excess water can drain out. If there are holes, the container should be placed on the grass or in another container, to catch the drainage.
2. Students will layer a thin layer of brown matter, such as dead leaves, newspaper, straw, or sawdust.
3. Students will then create a thin layer of green matter, such as grass clippings, or other green plant matter. (You can add food matter, but you should only do this if it will be stored outside and can be protected from animals.)
4. Students will place various products in both bins, including paper, a piece of a regular plastic bag, a piece of a biodegradable plastic bag, a piece of a plastic water bottle, as well as cardboard. These items should be in very small pieces, since the compost pile is small, about the size of a deck of cards.
5. Top it off with a thin layer of manure or topsoil. This will add the microorganisms that will speed the decomposition process.
6. Sprinkle with water.
7. Keep outside, at least during the day.
8. Students should record the matter they add to the pile.
9. Students should periodically check the pile to check the temperature and see if there are any changes. (The temperature should rise as the materials decompose.)
Closure: Students will note that some things decompose, or break down, in the compost pile, but other things don't change.
Student Activity 6
Objective: Students will calculate the number of days it would take to fill up the room with discarded paper.
Essential question: How much paper would we be sending to the landfill?
Procedure:
1. Teacher will secretly collect the paper discarded by the students in the classroom. He or she could also include a daily newspaper to speed up the simulation.
2. When the teacher believes there is enough paper to fill a rectangular cardboard box, he or she will stop and tell the children what he or she has done.
3. The class will calculate the volume of the classroom from measurement and the formula for volume. Then the class will calculate the volume of the box. By dividing the classroom volume by the box volume, they will determine how many boxes it would take to fill up the room with paper. If the box took 2 weeks and the number of boxes required to fill the room is 100, then the class can reasonably conclude that it would take about 100 multiplied by 2 or about 200 weeks to totally fill the room with paper. (A further conclusion could be determined to estimate the amount of days to fill up the room if all the paper in the school were added. Divide the total number of boxes by the number of rooms in the school, say 100 boxes needed to fill the room, divided by the 20 homerooms in the school, which would equal 5 multiplied by the 2 weeks and it would take only 10 weeks for the whole school to fill up the room with discarded paper!
Closure: We throw out a lot of paper and it takes up a lot of room.
Student Activity 7
Objective: Students will observe and test plastic bags in the air and water.
Essential Question: How do plastic bags create problems in the environment?
Procedure:
1. Students will take plastic bags outside.
2. Students will let go of the bags and see what happens to them.
3. If there is a wetland available, students can release bags into the water and observe.
4. Students should make sure they retrieve the bags after the observations.
5. Class will discuss the observations and make conclusions of the effect of plastic bags in the environment.
6. Teacher will show internet pictures of the Pacific Garbage Patch.
Closure: Students will see that plastic bags are aerodynamic in the environment and float in the water. They will make conclusions regarding the implications of this in the environment.
Student Activity 8
Objective: Students will conduct a taste testing of bottled and tap water among the school students and make conclusions based on the test.
Essential Question: Which water tastes better, bottled water, filtered tap water, or regular tap water?
Procedure:
1. Students will conduct a blind taste test of 2 different kinds of bottled water, tap water filtered with a simple pitcher filter, such as Brita, and unfiltered tap water. They will use paper cups that are not identified, except as A, B, C, and D.
2. Students will conduct the testing in other classrooms in the school.
3. Students will compile the data and discuss it. Students will create a poster bar graph.
4. Students will publish the graph for the school.
Closure: Students will make conclusions based on a taste testing between bottled and tap water.
Student Activity 9
Objective: Students will see how paper is made.
Essential Question: How is paper made?
Procedure:
1. Students will either watch the video online at the Glatfelter, Inc. website: http://www.glatfelter.com/learning/tour_pop_up.aspx, or by obtaining a CD from Glatfelter, or take a field trip to a paper mill, if that is available. Each source shows the steps in making paper.
Closure: Students will discuss the paper making process.
Student Activity 10
Objective: Students will observe the effects of melting different plastics together.
Essential Question: What happens when plastics are melted together?
Procedure:
1. Teacher will ask the students to bring in a variety of plastics. They should have various recycling codes, including 1, 2, 4, 5, and 6.
2. Teacher will cut samples from 2 plastics for each demonstration, heat them in an aluminum foil pan on a hotplate to about 300ºF. (Be sure you are in a well-ventilated area!)
3. Teacher will use a glass or metal rod to stir the melting plastic.
4. Students will observe the melting plastic and see how they mix.
Closure: Students will see that the plastics resist mixing together.
Student Activity 11
Objective: Students will recycle paper and compare recycled paper to paper that has not been recycled.
Essential Question: How is paper recycled? How does recycled paper compare with original paper?
Procedure:
1. Students will shred newspaper.
2. Students will mix up the shredded newspaper with water, using a mixer.
3. Students will pour the mixture onto a screen.
4. Students will allow the screen to dry overnight or use a blow dryer to speed the process.
5. Students will compare the recycled paper they created, along with other samples, (i.e. napkins, paper towels) to paper that has not recycled, using a hand lens, observing the fibers in the different examples.
6. Do you notice a difference between the two examples? What do you notice?
Closure: Students will see that paper can be recycled, but they will notice the difference between the recycled products and the original products.
Student Activity 12
Objective: Students will compose a persuasive essay letter to someone who could help reduce pollution or production of plastic or paper products. In addition, students will initiate a recycling project in the school or community.

Comments: