Strategies and Activities
This unit will primarily focus on recycling batteries, basic concepts of states of matter, electricity, and the electrochemistry of a battery. I will convey physical science concepts through hands-on, minds-on investigations with labs. The lessons will be broken down in five sections incorporating investigative labs after each concept presented. The initial lesson will be presented with a short video clip from a current event that was recently in the news. This will be projected via Smart board and a small discussion will follow. Finding out about my students' prior knowledge will better help me to provide them with the pertinent background knowledge to be successful. New concepts and vocabulary terms will be introduced and used as the unit unfolds.
Lesson One
This lesson starts with an introduction of recycling. The students will use their interactive notebooks and will work in groups of six to brainstorm what recycling means to them and ways they recycle at home, school, and in their community. Teams will also discuss potential hazards of not recycling. Pros and cons of recycling will be divided in the notebook. Teams will take turns and present their brainstorming ideas to the class using the Smart board. This pre-assessment of what knowledge the students have will determine how much time will be focused on the background information such as history, components, what a battery is, applications, and recycling. The students will discover that science is a way of using what we know to find out things we don't know. They will discover ways to solve problems scientifically.
Objectives:
*Students will explain the ways they recycle.
*The students will discuss the pros and cons of recycling.
*Identify the hazards associated with not recycling.
The teacher will present background information on the statistics of batteries that end up in landfills. The class will then estimate how many batteries they use a day. The class will calculate how many are used per week, month, and year. Hazardous materials will be discussed that are leached out of batteries into the ground. The students will discuss the products they know that are successfully recycled into something else.
Teamwork assignment:
The history of the battery will be presented in a timeline that will be copied and placed in the students' interactive notebooks. Students will work in teams to review the history of a battery. Other inventions and discoveries will be discussed associated with the time period. Benefits of a portable energy source will be presented. Teams will present sections of the timeline to the class.
Lesson Two
What is a battery? How does a battery work? Using the Smart-board, I will record student answers of what they know. I will then present the diagram of the cross section of a battery. The students will cut and paste a copy of a cross section of a battery in their interactive notebooks and label each component part. Essential questions will include: what did the first inventors use to make a battery? After a brief discussion, common metals will be presented and conductivity of such metals will be explained.
So how does charge flow from a battery through a light bulb? Every electrical circuit has some basic parts. Students will investigate, in the lemon battery investigation, some simple ways those parts go together to complete closed path called a circuit.
Students will learn through scientific problem solving by using an experiment, model, or observation to gather information to see whether their hypothesis is correct. Sometimes you need to alter your hypothesis, and sometimes you discover that the initial hypothesis is wrong. That's not failure. It's progress, because you now know you can make a new hypothesis and test it. In the lemon battery investigation, students will use observation to form a hypothesis.
Lesson Three
Investigation 1 Lemon Battery Lab
We will start by making a lemon battery to demonstrate electrochemistry, using zinc and copper nails, wire, and a bulb. The students will identify the electrodes of the closed system. Copper is the cathode and is positive. Zinc is the anode and is negative. The students will sketch what they will discover in their lab booklet which shows the path of Zn losing electrons and traveling to the other electrode. The H + ions from the electrolyte citric acid in the lemon has lots of hydrogen ions with a + charge. These H + ions take electrons that were deposited in the copper electrode: 2H + + 2e - => H 2. Electricity is produced because the loop is closed. By closing the loop, this allows the electrons to flow. An extension of the lab will be to have each lab group of six students chose another battery source from a variety of fruits and vegetables from the lad distribution area. I will facilitate the full inquiry technique. In full inquiry, students will be presented with a question. From this, they will design another investigation that they can conduct to answer the question. Students will have the opportunity to reach beyond what was demonstrated and work with their lab groups to discover by use of inquiry what will power their device. After each lab, the students will have the opportunity to gather their results, analyze them to draw conclusions, and present their findings to others. I will refer back to states of matter to assess what the students learned. Students will calculate if the lemon generates approximately 0.9 volts x 0.0003 amps = 0.00027 watts. The formula is volts x amps = watts.
Lesson Four
Why recycle batteries? What are the hazards? How can citizens recycle batteries? What can be done? Students will work in teams to research the effects of hazardous waste on humans and the environment. Each team will present their findings to the class and make the realization that batteries can cause harm to the environment if not recycled properly. The students will present ways they think can help in recycling of common household batteries, rechargeable batteries, and lead-acid batteries in their community.
Investigation 2 Solid or Dissolved Solution: Which will conduct Electricity?
The Periodic Table will be introduced and the students will focus on metal, non-metals, and noble gases. I will demonstrate that solids such as table salt (NaCl), Epson salt (MgSO 4), and baking soda (NaHCO 3) do not conduct electricity. The students will then assist in dissolving the salt, Epson salt, and baking soda in water (H 2O) to see if this "dissolved" solution conducts electricity. They will realize that solids prohibited the ions from flowing and the dissolved solution had free flowing ions, in an electrolytic solution. They will learn to write an equation such as:
MgSO 4 + H 2O -> Mg 2+(aqueous) + SO 4 2 -(aqueous).
Lesson Five
Investigation 3 Air Battery (alternate lab)
Students will be introduced to the Iron Science Teacher from the Exploratorium and view national fellow Lesia Whitehurst (Exploratium.edu) take on the challenge with her students on developing an aluminum air battery paper. I will pause the part before the students try to make the motor work. The students will then work in lab groups to develop their own battery with aquarium charcoal, saline saturated paper towel, aluminum foil, wire leads and a device of their choice from a supply of motors, light bulbs, clocks, or fans to power. The students will then make a saturated NaCl solution in a small bowl. Then they will rip off a piece of aluminum foil approximately 30 x 15 inches, place the saturated doubled over paper towel over the foil, next gently pour over approximately ¼ of aquarium charcoal over the paper towel. Place one end of a lead wire in the center of the charcoal and the other lead should be clipped at the end of the foil parallel to the first lead wire. Keep in mind that the internal lead should touch only the carbon inside. Remember to keep the carbon from touching the foil. Gently fold like a large burrito. The students will then attach both leads to the motor they wish to operate. The lab teams will record what is happening. Gently push down on the foil wrapped battery and record what is happening. The motor or device should work. Point out that the outside aluminum electrode is the low voltage electrode; therefore, it's the source of electrons. The reaction that powers the battery occurs between the aluminum foil and oxygen from the air. Explain that the activated charcoal has many gas pockets giving it a large surface area which provides the oxygen to the electrode; thus, the reaction with aluminum occurs in an aqueous solution. The maximum current delivered is determined both by the voltage produced by the battery and the internal resistance of the battery. The charcoal is the conductor; thus, the resistance decreases as the charcoal pieces are pressed together.
Lesson Six
What effect do battery metal toxins have on the ecosystem? What acid levels are leached in the soil and water? Students will be given questions and form a hypothesis to formulate an answer. The students will conduct a pH water lab investigation.
Investigation 4 pH Water Test
The students will watch me place an opened battery that I will open prior to lab, in a container of water and make predictions and form a hypothesis of how the battery will affect the pH. Each lab team will use a water testing kit containing phenol red. The students will use the clear container and read the color change indicating the pH level; the students will then compare the levels to a pH scale determining what pH levels are acceptable. We will discuss the acid, base, and neutral levels and the hazards of the bases from the batteries in landfills and the problems associated with it.
Investigation 5 Hazardous Waste Ecosystem
Lesson Seven
The final lab will be the culminating activity where students design an ecosystem with a small aquarium with plants, soil, worms, insects and a leach bed landfill system to simulate their community. Each lab team will have a control and an experimental ecosystem for this experiment. The variable will be the battery water. The battery contaminated water will be used to water their ecosystem. The students will observe and make record their results. The students will make a final poster board or Smartboard presentation to the class on their results. Students can record the pounds of batteries collected and calculate the benefits of reducing the landfills.
Extension Activity
Students can spearhead a recycling program in their neighborhoods. They can coordinate the education of it by holding small town talks and invite a member from the EPA or other environmental agency to speak. The students can make posters, flyers, and coordinated collection of containers or large re-sealable bags with instructions of where to take the filled containers for recycling.
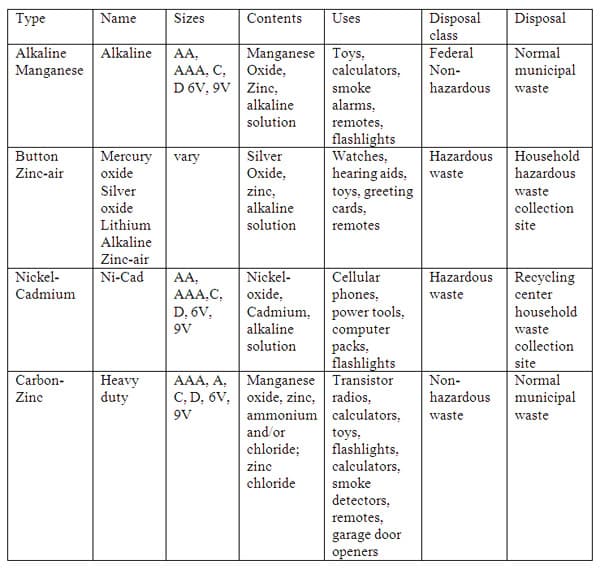
Primary Batteries (figure 1) 25
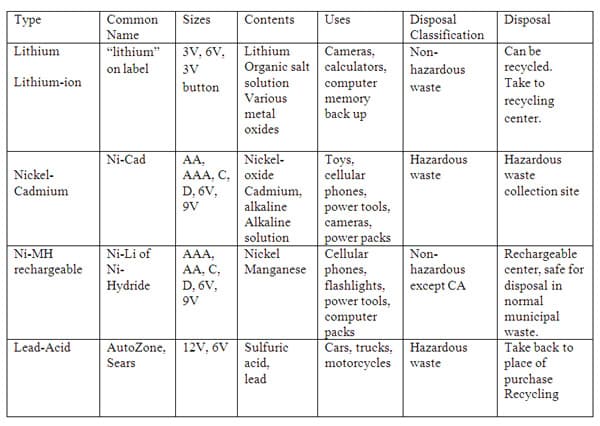
Secondary Batteries (figure 2) 26

Comments: