Activities
Students will conduct body system experiments using the science inquiry process of questioning, planning and conducting investigations, using appropriate tools and techniques to gather data, thinking critically about the relationships between evidence and explanation, and communicating the results.
Activity 1: Digestion
HYPOTHESIS: The chemical pH is a property that tells whether the liquid is an acid or a base. Acids have free hydrogen ions and a pH of 1-6. Bases have free hydroxyl ions and a pH of 8-14. Distilled water is neutral with a pH of 7. What do you think might happen to the pH if an acid and a base are mixed together?
MATERIALS NEEDED
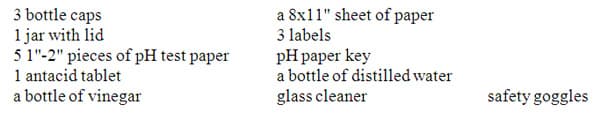
PROCEDURE
1. Label each of the three bottle caps: distilled water, vinegar, and glass cleaner.
2. Put a small amount of the appropriate liquid in each cap.
3. Test the pH of each and record it in the following table.
4. Which one is the acid? ____________________
5. Which one is the base? ____________________
6. Test your hypothesis by pouring one into the other.
7. Shake the cap gently to mix the two.
8. Test the mixture with a clean piece of pH test paper and record the results in the table.

9. Fill the jar about half way with vinegar. The jar will simulate your stomach and the vinegar will simulate the hydrochloric acid in your stomach.
10. Some people get stomach aches and take antacid tablets to help. Wrap an antacid tablet in a piece of paper and carefully stomp on it until it is crushed. We will see why.
11. Pour the antacid into the jar, put the lid on, and shake.
12. Use the pH strip to test the pH level: ________
13. What happened to the pH in the "stomach"?
CONCLUSION:
QUESTION: What part of the digestive process did you simulate by stomping on the antacid?

Comments: