Background
What is energy?
In 2003, Rice University professor and Nobel Prize winner Richard Smalley made a list called "Top Ten Problems Facing Humanity Over the Next 50 Years." 10 In his list, he put energy as the number one problem. If energy is arguably the biggest concern for today's society, then it is important to understand the meaning of energy. It can be a mysterious word to some because it is an abstract entity that is elusive and not concrete. In the scientific context, energy is defined as the ability to produce change or do work. Energy can produce light, heat, motion, sound, growth, and can power technology. 11 Just as our body can take in food and convert food into energy that makes our body function, the world requires ways of capturing, storing, and converting energy to function. Developing new technology to efficiently make high value energy such as electricity and fuel using natural resources is a fast developing field in energy research.
What are the sources of energy?
There are two general categories for sources of energy: non-renewable and renewable energy. Non-renewable energy sources, as the name suggests, cannot be replenished once the energy source is used up. The ultimate source of non-renewable energy is fossil fuels. Fossil fuels come from plant and animal matter that have been decayed and processed naturally over millions of years. They are stored under the surface of the earth and must be extracted out of the ground. These energy sources include coal, petroleum, and natural gases. 12
On the other hand, renewable energy sources are considered better options than non-renewable energy sources because they can be replenished and we will never run out of them. 13 Some sources of renewable energy are wind, solar, hydropower, and biofuels. Wind power can be harnessed through wind turbines and used to make electricity. Solar energy is power that comes from the sun's rays that can be captured by photovoltaic cells. The solar energy can be converted into heat or electricity. Water can also supply energy through dams to capture the energy of flowing water to generate electricity. Finally, plant and animals can be turned into biofuels by simply burning them or turning them into liquid fuels such as biodiesel. 14
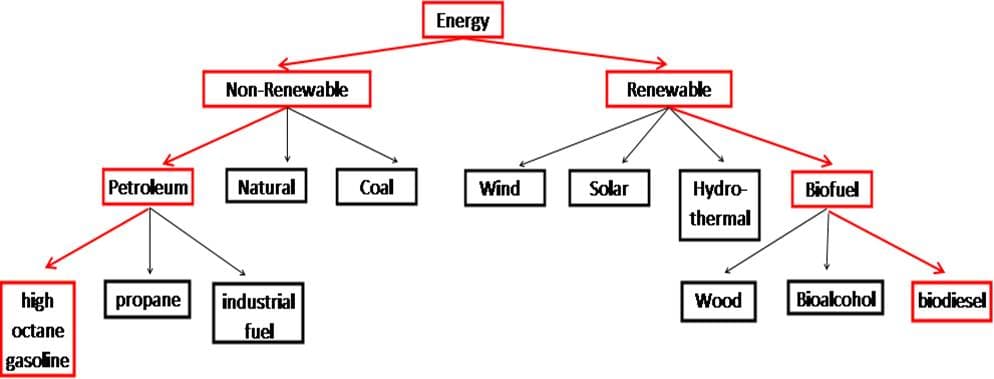
Figure 1: The flow chart shows a general overview of the different types of energy sources. The red portions of the flow chart outline the specific types of energy that will be the focus of this unit.
It is evident that there are many avenues we could take to capture energy for our use. It is impossible to go into detail about all the different energy sources as they are too numerous. For this unit, the focus will be on two sources of energy used specifically for transportation. Figure 1 is an outline of the different types of energy. It is not a comprehensive list by any means. This unit specifically compares two sources of energy. High octane gasoline made from petroleum will be the focus of non-renewable energy and biodiesel fuel made from plant seeds as the renewable energy.
High Octane Gasoline
Currently, 28% of the total energy production in the United States is dedicated to transportation. It is the second biggest area of energy consumption behind electrical power at 40% consumption. 15 Most cars use unleaded high octane gasoline as fuel. High octane gas is a mixture of at least 500 various hydrocarbons ranging from 5 to 12 carbon atoms. This mixture is created from crude oil which has been processed through many refinement steps. 16 When ignited, the gasoline releases a lot of energy which is used to move the car. The high octane gasoline goes through a combustion reaction which breaks down the gasoline, as it reacts with oxygen, into carbon dioxide and water. To put things into perspective, there were over 244 million cars registered in the US in 2008, compared to 79,000 registered cars in 1905 when the first affordable car was introduced. 17
Crude oil, also known as petroleum, is a type of fossil fuel. There are different grades of crude oil and each grade is used for different purposes. Some of the perhaps surprising uses of crude oil are clothing, vitamin capsules, tires, and even toothpaste. Raw crude oil is of little use but can be refined to make numerous useful products ranging from asphalt for construction to plastics. There are three major refining processes to change crude oil into the desired product. These three steps are: Separation, Conversion, and Purification. 18
In the separation stage, raw crude oil is distilled and separated by taking advantage of differing boiling points of the components of raw crude oil. Some examples of components with low boiling points are butane and propane and examples of high boiling point components are industrial fuels and asphalt base. The crude oil is placed in a distillation column so that compounds with lower boiling points will become vapors and rise to the top of the distillation column while those with high boiling points will remain at the bottom. 19 In this way, the crude oil mixture can be separated into its different components.
Some components can be used after the separation stage but others will need to go through a conversion process to convert low value oil into high valued ones, especially gasoline. This is done by taking the low-value long chain hydrocarbon oils and breaking the chains to make smaller molecules that are of higher value. Some examples of these higher value oils are kerosene used as jet fuel, gasoline, and diesel. 20
In the final step, the converted components must be purified. The main purpose of this step is to remove sulfur. This is done by hydrotreating in which the components react with hydrogen under heat and high pressure in the presence of a catalyst. The sulfur in the converted components is extracted, resulting in hydrogen sulfide (H 2S) and the now desulfurized product. The product is now ready for use and the hydrogen sulfide is further refined into elemental sulfur and water. 21
Biodiesel
One of the many possible renewable energy sources that are currently being explored is biofuels. Biodiesel is a specific type of biofuel can be made by recycling used frying oil such as corn oil from the kitchens or it can be harnessed from plant seeds. Some of the most common sources of biodiesel come from rapeseed oil, sunflower oil, and soybean oil. 22 The oils are transformed into biodiesel through a chemical process so that they can become fuels to power vehicles.
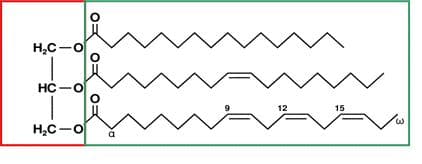
Figure 2: Triglyceride structure. The red box indicates the glycerin portion and the green box indicates the fatty acid chains. 23
The structure of vegetable oil used to make biodiesel is called a triglyceride. It contains a glycerin molecule that is attached to three fatty acid chains as shown in figure 2. Vegetable oil cannot be used directly in a diesel engine because it is too viscous and so it must be chemically altered into biodiesel. This is done through a process called transesterification where the glycerin from the vegetable oil is replaced by methanol, thereby producing three fatty acid chains. These separated fatty acid chains can now be used as biodiesel. 24
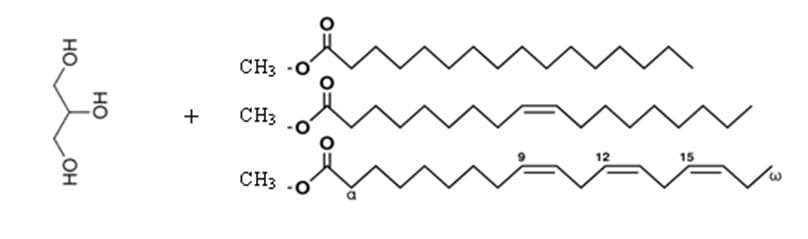
Figure 3: Production of biodiesel. The left side shows the structure of glycerin. The right structure shows three separate fatty acid chains that are now called biodiesel.
The process of making biodiesel is relatively simple. One of the benefits of biodiesel is that it can be made in large quantities in the industry or in small quantities at home. The first step to making biodiesel is to combine methanol with sodium hydroxide to make sodium methoxide (NaOCH 3). The sodium methoxide is the added to the vegetable oil to remove the glycerin and release the fatty acid chains as esters. Glycerin is denser than the fatty acid esters and will settle at the bottom of the mixture and the biodiesel can then be isolated. 25 The biodiesel is then purified using water to wash out any excess glycerin and then can be used as a fuel source.
Energy Reactions
Chemical reactions occur when molecules change their chemical compositions. There are five general reaction types: single displacement, double displacement, decomposition, synthesis, and combustion reactions. Energy from the both high octane gas and biodiesel are released through a process called combustion. A combustion reaction occurs when an organic molecule made of carbon, hydrogen, and oxygen atoms react with the oxygen molecules in the air. A spark is needed to overcome the activation energy needed to start the reaction. Once it starts, the reaction releases energy and forms carbon dioxide and water molecules as the products. 26 The general chemical equation is written below.
CxHyOz + (x + y/4 – z/2) O 2 —> x CO 2 + (y/2)H 2O(equation 1)
When expressing reactions as a chemical equation, it should also be expressed in a way that follows the law of conservation of matter which states that matter cannot be created or destroyed. In chemical equations, this is shown by balancing the chemical equation. Both the left side (reactants) and the right side (products) of the arrow must have the same number of atoms. The equations below demonstrate the combustion of octane and a biodiesel fuel (the middle molecule on the right side of Figure 3 is used).
2 C 8H 1 8 + 25 O 2 —> 16 CO 2 + 18 H 2O (equation 2)
C 1 9H 3 6O 2 + 27 O 2 —> 19 CO 2 + 18 H 2O (equation 3)
Balancing equations can be difficult because there is no mathematical equation that will provide the correct answer every time. However, it is possible to follow steps to get to the correct balanced equation. The first step is to write out the chemical equation with correct chemical formulas for the reactants on the left and products on the right. Then identify the atoms in the most complex substance and balance those atoms. The atoms that occur as free elements should be balanced last. Finally, if any coefficient in the balanced equation is a fraction, then the entire equation must be multiplied by the denominator of the fraction to make sure whole number coefficients are used. 27
Stoichiometry of Energy Reactions
Once an energy reaction is written out as a balanced chemical equation, it is then possible to analyze the relationship between what is put in and what comes out of the reaction. In particular, the interest in energy reactions is the output of carbon dioxide molecules, the greenhouse gas of interest for this unit. The coefficients for the balanced equations show the mole relationships between the molecules involved in the reaction. From the examples above, we can see that two molecules of octane will produce 16 moles of carbon dioxide. The number of moles of carbon dioxide produced is 8 times greater than the number of moles of octane that is burned. In comparison, biodiesel fuel is a larger molecule and produces 19 moles of carbon dioxide for one mole of biodiesel.
Not only can we do these calculations by looking at molecule to molecule relationships between products and reactants, it is more practical to discuss this reaction in terms of the mass. Octane has a molar mass of 114.23 g/mol and carbon dioxide's molar mass is 44.01 g/mol. As an example, if 500. grams of octane fuel were burned, it is possible to calculate the mass of carbon dioxide that will be produced using their stoichiometric relationship. 500. grams of octane will release 1540 grams of CO 2 into the atmosphere. The molar mass of biodiesel is 296.50 g/mol so it is more than twice as heavy as octane. Using the same example, 500. grams of biodiesel fuel will release 1410grams of carbon dioxide.
This can be counter intuitive because equation 1 shows that 16 molecules or moles of carbon dioxide is produced compared to equation 2 which shows 19 molecules or moles for biodiesel. This is a good opportunity to point out the difference between mole to mole relationship which describes quantity with grams to grams relationship which describes mass. Because biodiesel is a larger molecule, 500. grams of biodiesel actually contain fewer moles than 500. grams of octane. As a result, the combustion of biodiesel produces less carbon dioxide than the same mass of octane gasoline.
Heat stored in chemical bonds
The reason why fossil fuels are such a precious source of fuel is that a lot of energy can be produced from a relatively small amount of it. The energy of the fuel source is stored within the bonds of the molecules. The energy released from fuel sources can be numerically calculated by looking at bond energies. Energy must be put in to break bonds but energy is released when bonds are broken. If there is a net release of energy, this reaction is exothermic and the numeric value is given a negative sign to indicate the release of energy. 28 If there is a net absorption of energy, this reaction is endothermic and the numeric value is given a positive sign.
Taking a look at the octane molecule from previous examples, students can calculate the energy needed to break the bonds and compare it with the energy released when the bonds of the products are formed. The reaction from equation 2 is written below in a way that shows the number of bonds broken on the reactant side and the number of bonds formed in the product side.
2 (18 C-H + 7 C-C) +25 (O=O) —> 16 (2 C=O) + 18 (2 H-O)(equation 4)
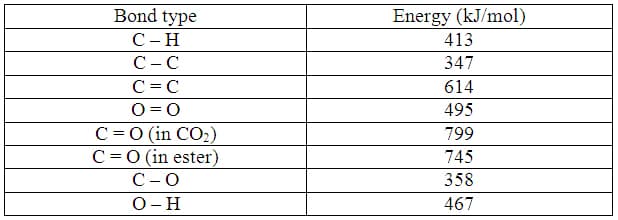
Table 1: The average bond energies for bonds that are broken or formed during the combustion reaction of fuels. 29
The energy of bonds broken in octane is calculated to be 32100 kJ while the bonds formed release -42400 kJ of energy. This means that -10300 kJ of energy is released for 2 moles of octane. This means that 1 mole of octane will release -5150 kJ and that excess energy is what we can use to power our cars. The same calculation can be done for equation 3, the combustion of biodiesel. A mole of biodiesel will release -11300 kJ of energy. In terms of quantity, biodiesel releases more energy per mole than octane.
As mentioned in the above paragraphs, it is also important to compare the energy content for equivalent mass as well, since octane and biodiesel do not have the same molar mass. This shows a clearer picture of the energy comparison because in real life, it is more practical to measure out masses of fuel than moles of fuel. Using the example of 500. grams of each fuel types, octane would release -22500 kJ and biodiesel would produce -19100 kJ of energy. In this case, octane releases more energy by mass.
So what is the point?
The Energy Crisis
Taking a look at energy consumption in units of British Thermal Unit (BTU) in the United States, energy use in 1949 was 31.982 Quadrillion BTU. Today, the country uses 97.301 Quadrillion BTU of energy. 30 This drastic increase in the amount of energy consumed in the United States has led to the push of moving away from non-renewable sources of energy toward renewable or sustainable energy sources.
Despite the data that show the significant increase in energy consumption, it has been argued that there is no need to fear running out of energy sources. As seen in previous energy crises, these crises are met with advancement of technology to meet the needs of the consumers. 31 Today's concern of energy is not the shortage of resources but the shortage of the rate of extraction of oil. 32
If there is indeed an ample supply of fossil fuels to sustain the population, then the next step is to look at the consequences of using the current amount of energy and figure out if the environment can cope with the current energy usage. The issue at the forefront of energy use is the increase of greenhouse gases that is causing global climate change. In particular, the combustion of gasoline and biodiesel both release carbon dioxide into the air which is one of the biggest contributors to the climate change. However, there are two key differences between the two energy sources.
As mentioned before, biodiesel is a renewable energy source while high octane gasoline is non-renewable. Furthermore, biodiesel is made using plants that are part of the current carbon cycle and, therefore, does not introduce new carbon dioxide into the atmosphere. Plants get energy from the sun and use that energy to perform photosynthesis. Photosynthesis is the process by which plants take sunlight, carbon dioxide, and water from the atmosphere to produce oxygen gas that is released into the air and sugar that is stored up in the plant (equation 5)
Sunlight + CO 2 + H 2O —> O 2 + (CH 2O)(equation 5)
(CH 2O) + O 2 —> H 2O + CO 2(equation 6)
(CH 2O) is an abbreviated notation for a carbohydrate. Animals depend on the photosynthesis of plants as a source of oxygen and sugar (for food). As animals respire, they give back to the plants by providing them with carbon dioxide needed for photosynthesis (see equation 6). 33 The burning of biodiesel fuel does not contribute more carbon dioxide to the atmosphere but converts the carbon that is already part of the carbon cycle from plant matter into atmospheric carbon dioxide. In other words, biodiesels do not use sequestered carbon and so it is actually considered a carbon neutral compound.
Burning fossil fuels also releases CO 2 into the air. However, the problem with carbon dioxide emissions arise because fossil fuels are sequestered carbon molecules. These carbon molecules have been buried deep under the earth and are not part of the atmospheric carbon cycle. The combustion of these fuels introduces new carbon dioxide into the atmosphere. The increased carbon dioxide prevents unwanted heat from escaping out of the atmosphere. The extra heat that is contained within the earth's atmosphere impacts the environment. 34 This effect is called global climate change.
Gasoline or Biodiesel: Which is better?
Although it is true that high octane gasoline is a big contributor to global climate change, it is still the preferred fuel for transportation. The perfect fuel that provides a large amount of clean energy that can efficiently be harnessed at low cost is yet to be discovered. Many other factors, such as economics of fuel and energy policies, must be analyzed to understand the reasons why people choose carbon contributing gasoline over cleaner fuels.
One of the biggest driving forces for gasoline is there is an ample supply of petroleum that can be converted into gasoline. New technologies that allow us to extract petroleum from sources that we once thought were impossible have opened up opportunities for petroleum production. For example, shale oil is sedimentary rock that can release petroleum-like liquids when heated. 35 Until recently, it was thought to be impossible to extract liquid petroleum from solid rock. However, this new discovery has opened up a new source of petroleum for us to use instead of just conventional crude oil.
In addition to the abundance of petroleum fuels, the cost of high octane gasoline is relatively cheap. 36 From an economic standpoint, it makes more sense to use high octane gasoline as fuel because it is relatively cheap ($3.99/gallon) compared to commercial biodiesel ($4.11/gallon). 37 It is difficult to encourage people to pay more for biodiesel out of their environmental concerns when gasoline is cheaper and more readily available at the gas pumps.
Another struggle for biodiesel is that its production is not efficient. In 2011, 9% of the US energy came from renewable energy and of the 9%, only 13% of it was used in transportation. 38 The challenge is that because biodiesel comes from plants, there must be enough plant material to make the fuel. In order for biodiesel to make a significant impact, energy could be produced by deliberately growing energy crops. However, this requires substantial amount of land and water. In addition, photosynthesis is an inefficient process that converts only 1% of the sun's energy into biomass. 39 In light of these challenges, biodiesel may be an impractical energy source.
The answer to the question of which is better depends on each person's own personal convictions and their needs. It is a difficult question to answer but also provides opportunity to weigh out the pros and cons of each side for students to make their own decisions. Of course these are not the only options for energy sources as mentioned in figure 1. Although there is a big push to find the one source of energy that will provide all the energy needs of the world, perhaps finding ways to use multiple energy sources in moderation is an option that should be explored as well.

Comments: