Appendix 4
Connecting to Chemistry:
Word bank: activation energy, atoms, broken, collision, endothermic, exothermic, products, reactants, releases
In a chemical reaction _________ just rearrange.
At the beginning of a chemical reaction we call the chemicals we start with _____________. There must be a ______________ in order for a chemical reaction to take place. The bonds of the reactant molecules must be _____________ which requires energy. The atoms are rearranged and then form new bonds which _____________ energy. The new chemicals that are produced are called the ______________.
Sometimes extra energy must be added to make a chemical reaction happen, this is called the ______________________.
At the end of a chemical reaction if you end up absorbing energy it is considered an ________________ chemical reaction.
At the end of a chemical reaction if you get extra energy being given off, usually in the form of heat or light it is considered an ________________ chemical reaction.
The Law of Conservation of matter reminds us that _____________________________.
There are multiple types of chemical reactions that can be identified. Match each type of reaction with the appropriate partner, then determine if they exemplify the law of conservation of matter or not. If not place the appropriate coefficients on the provided lines to satisfy the law.
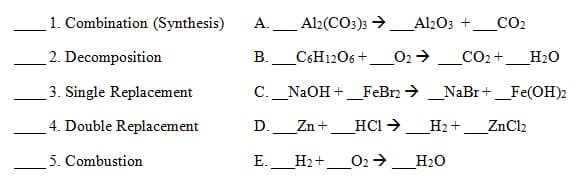
**Bonus: Reaction B is incredibly important to human life, what process does this reaction represent?
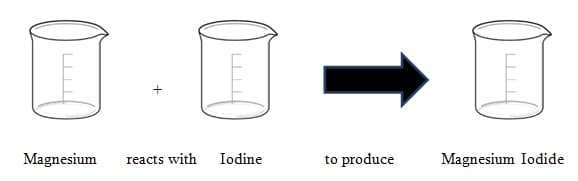
Using the following reaction show at the particle level how it exemplifies the law of conservation of matter.
What happens when you have an allergic reaction?
Allergic Reaction video: http://health.howstuffworks.com/human-body/14063-body-invaders-allergies-and-dust-mites-video.htm
How can you relate what happens during an allergic reaction to a chemical reaction?
The chemical reactions we have looked at in class usually have less than 10 atoms in each reaction. Why do you think we did not use the chemical reactions that happen during an allergic reaction?
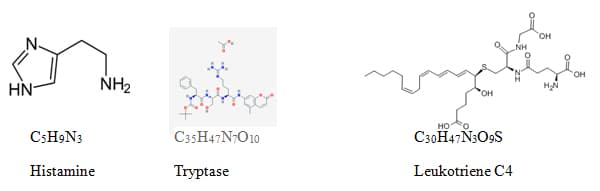
Even though we use the most basic chemicals there are during class, the process of a chemical reaction still remains the same. In your own words describe the process of a chemical reaction and how this is similar to how microbes interact with our bodies and our environment.
Be the first to comment on this unit!

Comments: