Classroom Activities: Design and Engineering
The intent of this unit is to inform students about the best practices in food preservation and storage. In class, students will use this understanding to design and build appropriate storage units in order to reduce bacterial or enzymatic activity in food and drinks. Even though this will be a unit for Chemistry, there is a lot of microbiology involved in understanding food spoilage due to bacterial, yeast, and mold. Food preservation techniques such as pasteurization and freeze-drying limit these interactions.
The activities noted below are modifiable to address different scientific content in a science class. The major themes of these activities are to expand upon the NGSS and our district’s aim to incorporate activities for students to demonstrate depth of knowledge (DOK) level 3 and 4. DOK levels are explained in “Implementing District Standards.” These standards are achieved when students demonstrate their understanding by designing a model or experiment to gather data that supports the physical science content.
Activity 1 – Writing Claims and Identifying Variables
The activity will require students to explore the interaction between ketchup (food item) and spoons made from different materials. As with all labs, students will begin by writing a claim. I moved away from using the word hypothesis to eliminate the first person reference. An example is provided to help students feel more comfortable with writing claims (e.g. The plastic and wooden spoons will not react with the ketchup). The protocol only presents a graphic of what the setup should look like but no instructions (Figure 1). This is to have students explore more openly with the material, thus pushing cognitive limits and encourage students to think and work beyond their comfort zone.
Each class is divided into teams based on the amount of materials available. Teams of three or four are ideal. If additional teams are needed, then each team may choose two different material spoons until all spoons run out. I would assign a few teams to setup the control to ensure there are enough containers to use for each team.
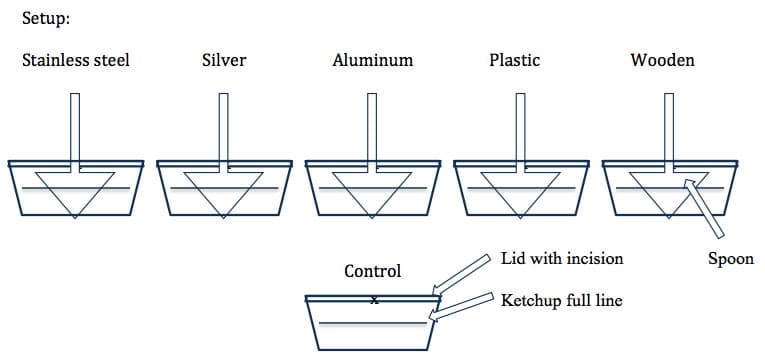
Figure 1. Setup provided to students during the beginning of the Ketchup Lab.
Some likely questions to arise during the activity:
- Should a line be drawn on the containers to show the initial amount of ketchup?
- Do we need to close all containers as they are done being used or all at the end?
- Does the spoon need to be fully immersed in the ketchup?
- When will we check the results?
All teams are instructed to place their setups in a location in the room so as not to be disturbed. A paper towel can be used to label each team’s setup. The best results occur when the containers are placed in a warm space of the room with some direct sunlight. The activity can also be done in a cool room with little direct sunlight, which may result in obvious tarnish of the silver spoons; however, no result will occur with the aluminum covered spoon. The other material spoons are inert and will result in no reaction with the ketchup.
I would then discuss the data tables and the two types of observations: qualitative and quantitative. Thereafter, the discussion questions are adaptable to one or more of the following topics: interactions between matter (physical and chemical changes) and chemical reaction/stoichiometry.
Activity 2 – Permeability of Plastic Wraps
Different types of plastic wrap have varied permeability. This activity allows students to determine what type of plastic wrap will prevent browning or oxidation of sliced fruits. To start this activity, students should observe and briefly explain the chemical reaction between oxygen gas and phenol compounds in fruit. Taking a sliced apple and leaving it unwrapped will provide a good visual for students to provide claims as to how or why the color changed from white to brown. Students are then tasked with determining which type of plastic wrap will slow down this oxidation process the most. With the inspiration from ScienceBuddies.org, students should design their own experiment of testing different fruits that are known to brown and different plastic wraps (e.g. low density polyethylene (LDPE), polyvinyl chloride (PVC), and polyvinylidene chloride (PVdC)).23
I have students make predictions on which type of plastic wrap and under what specific conditions would create the least amount of oxidation of the fruit. Their research includes some information about different types of plastics and which fruits brown due to oxidation. Students would write their own list of materials, procedures, and data table prior to being able to conduct their experiment. Their procedures should include how to prepare their fruit samples, the conditions in which the fruit is stored, and the length of time for observations. Once their experiment is completed, students need to analyze the results and updates to the original procedure with rationales as to how these updates improve the experiment.
Possible variations in setup may include:
- Placing plastic wrap directly on the fruit’s open surface.
- Using a vacuum seal method to remove oxygen between the fruit and plastic wrap.
- Placing food in a plastic container and then using plastic wrap to cover the container.
To promote creativity in the ways plastic wrap is used, I limit only two teams using the same setup or procedures. A third team with a similar setup would be asked to use the plastic wrap in a different way. Their final submitted team assignment is a portfolio including all revisions of their procedures and data.
Activity 3 – Designing an Energy Efficient Icebox
One major method of food storage is refrigeration. In an emergency or natural disaster, electricity may be out for long periods of time and our perishable food may spoil at a quicker rate. This activity is for students to design and build an icebox that can keep highly perishable foods, like fresh herbs and dairy products, from spoiling without the use of electricity. Understanding the first and second laws of thermodynamics will help students choose their materials and strategize how to limit heat energy from moving to and through the food.
The icebox design should include materials not already made to be used as a cooler (e.g. foam box). Students should use materials found in and outside their home when designing their iceboxes. The determination of the efficiency of their iceboxes will mainly be qualitative. Although calculating the efficiency in energy retention by the icebox is possible, a straightforward qualitative analysis is sufficient in determining the effectiveness of their icebox design. Each team of students would store the same perishable food in their icebox and compare the freshness of their food to ones stored in a refrigerator. Students may also use data gathered from other teams’ iceboxes to see the similarities and differences in efficiencies in preventing spoilage of their food.

Comments: