Scale: Measuring Microscopic Matter
A scale is a tool that humans have made to measure things. There are many different scales but they all measure physical traits, like distance, weight, and size. Scales have names which define the units (standardized smaller parts) used with that scale. Scales are very important for describing things clearly. For example, when a contractor is making a building, accurate measurements are need so the walls and doorways are straight. When baking cupcakes, careful measurements ensure the results are tasty and fluffy every time.
|
Metric |
Customary |
|
|
Length |
meter |
inches feet yards miles |
|
Weight/Mass |
gram |
ounces pounds tons |
|
Volume |
liter |
teaspoon tablespoon ounces cups pints quarts gallons |
|
Temperature Water Freezes Water Boils |
Celsius 0° 100° |
Fahrenheit 32° 212° |
Table 1: The simplicity and logic of Metric units are contrasted with the subjectivity of the Customary system.
The units within a scale are fixed and discrete (inches, millimeters, miles, gallons). They are specific, measurable and inarguable. What is arguable is the quality of various scales. In the United States, we use “Customary Units” which have a more historical than scientific origin, while 96% of the rest of the world and all of the scientific community use the Metric System of measurement.
Customary Units are based on a very human scale. For example, an inch is based on the width of a man’s thumb and the foot was based on the average length of a man’s foot. These units of measure were convenient in their historical context but from a scientific perspective they are inefficient because they do not easily convert from one level of degree to another. The Metric System is much easier to use because it is based on units of 10 (deci-), 100 (centi-), 1000 (milli-/kilo-), etc. This makes it easy to convert from one level to another.
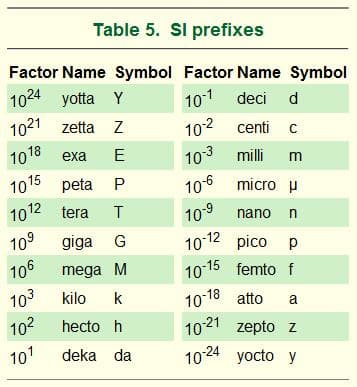
Table 2: Common prefixes, symbols and exponents of the Metric system
Microscopic particles are tiny but they still vary vastly in size. Exponents, also known as powers of ten, are way of writing very large (or tiny) numbers. Essentially, the exponent (little raised up number) tells you how many zeros come after the base number (ex. 109 =10,000,000,000). Common measurement units at the microscopic level include the Angstrom (Å) micrometer, and nanometer. Angstrom equals one hundred-millionth of a centimeter, 10-10 meter, or 0.1 nanometer. The angstrom and multiples of it, the micron (104 Å) and the millimicron (10 Å), are also used to describe extremely small particles.

Comments: