The Curriculum Unit
The curriculum unit is arranged in three parts. Part one presents an overview of the processes and mechanisms of normal bone development and remodeling. There is a specific focus on the roles of the various cell types and cellular components. Part two is an examination of established, novel, theoretical restorative therapies for bone and joint conditions. Part three is an exploration of the risks, consequences, and ethical considerations related to various restorative therapies for bone and joint conditions.
Part One - Overview of the processes and mechanisms of normal bone development and remodeling
Previous parts of the larger skeletal system unit will have introduced students to the various processes that result in the bones that support their bodies and allow them to move among other functions. In short, before engaging with part one of the curriculum unit, students will understand that bone formation begins early in fetal development and includes intramembranous ossification (the osteogenic process by which the clavicles and most of the skull form) and endochondral ossification (the osteogenic process by which long bones like the humerus and irregular bones like those comprising the vertebral column and pelvis are formed) (Shier, Butler, & Lewis, 2013).
Students readily observe and embrace the idea that through infancy, childhood, and adolescence bones grow in size and strength; that much is plain to see. What is less obvious to students, is that adult bones are not inactive, inanimate objects waiting to be acted upon by muscles or other organs. The bones of an adult, indeed all bones, are dynamic living tissues that are constantly undergoing changes in response to environmental and/or mechanical stressors (Shier, Butler, & Lewis, 2013).
Bone remodeling is an essential process for maintaining bone strength and mineral homeostasis (Shier, Butler, & Lewis, 2013). Bone remodeling allows the body to repair old damaged bone and make structural adjustments in response to the loads the bone must support. The process on bone remodeling is intricate and coordinated yet evokes a certain simplistic elegance when considered as a whole. Understanding this process and the roles played by the specialized bone cells involved (osteocytes, osteoclasts, and osteoblasts) is critical to begin answering the question “How can humans take advantage and manipulate bone physiology to enhance bone-related medical interventions?”
The following imperfect yet effective analogy will allow students to grasp the phases of the bone remodeling cycle (activation, resorption, reversal, formation, quiescence). Bone remodeling is akin to the process of remodeling a worn damaged house and the various people involved can be loosely analogized to the osteocytes-mature bone cells, osteoclasts-bone removers, and osteoblasts-bone builders). To begin, phase 1 – activation, the home owner must signal to the construction company that the building is in disrepair and in need of remodeling. In this way the homeowner is not unlike an osteocyte. Osteocytes are fully differentiated osteoblasts trapped within the bone matrix. In response to stresses, for example a mechanical load on the bone, osteocytes in the region of the load respond by releasing signal factors. It is through these signal factors, that osteocytes regulate osteoclast and osteoblast activity and thus coordinate bone remodeling (Knothe Tate, Adamson, Tami, & Bauer, 2004). As the process of remodeling a home involves an initial crew with particular tools for stripping away much of the old structure, so does phase 2 – resorption, involve removing the old damaged bone. In this phase osteoclasts employ acids and enzymes to remove the organic and mineral components of bone (Knothe Tate, Adamson, Tami, & Bauer, 2004). It is only after the old parts are removed that there can be a shift in process. This is comparable to phase 3 – reversal, in which the osteoclasts leave the remodeling site. In phase 4 – formation, a new crew with different tools arrives to begin the new construction. Osteoblasts secrete a collagen matrix in the resorption pit and control its mineralization forming new bone (Knothe Tate, Adamson, Tami, & Bauer, 2004). After formation the system rests in phase 5 – quiescence, until such a time as there is another signal for activation. When students are able to identify factors in the construction scenario that might improve or speed-up results (e.g. more or more active construction workers, more or better tools, more or better raw materials), so too can students begin to contextualize the roles of growth factors like bone morphogenic proteins.
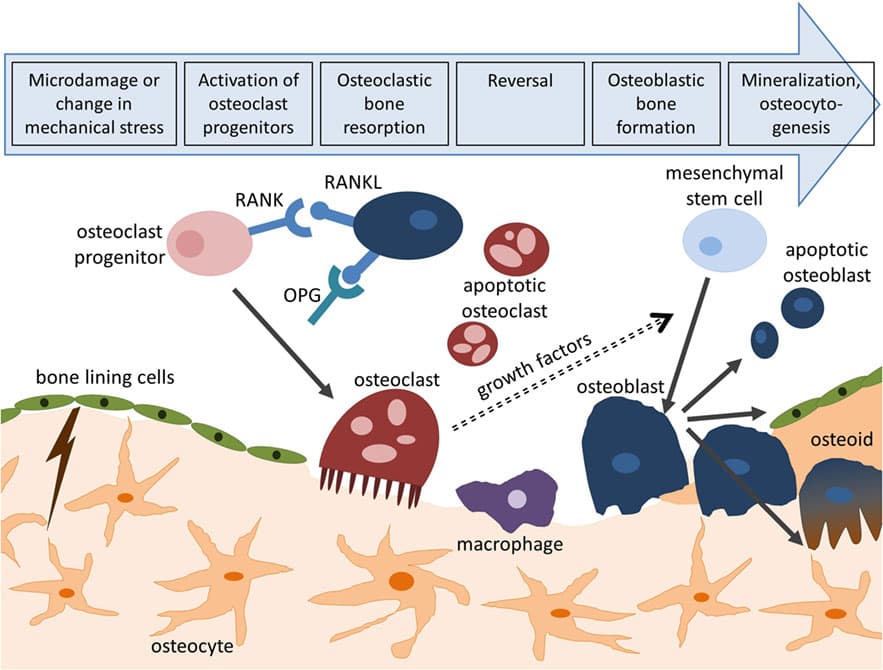
Figure 1 - Bone Remodeling Cycle
Part Two – Augmenting Bone Regeneration: Restorative Therapies (Established, Novel, and Theoretical)
Both in the United States and worldwide, the need for effective and efficient restorative therapies for bone loss is substantial. Bone loss or damage due to musculoskeletal disease or injury has significant medical and economic ramifications on individuals and society more broadly. The American Academy of Orthopedic Surgeons periodically catalogues these impacts in The Burden of the Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost, most recently in 2014. Key takeaways from the most recent volume of Burden included the following: musculoskeletal conditions are associated with nearly 600 million physician visits, 400 million visits to non-physician health providers, as many home health care visits, and more than 20 million hospital admissions. In the most recently documented 2-year period, persons with musculoskeletal conditions averaged nearly 21 prescriptions per person filled for these conditions, totaling more than 2 billion prescriptions. In the same period, the aggregate economic impact of musculoskeletal conditions was near $800 billion (Initiative, 2014). Worldwide, osteoporosis alone accounts for 9 million fractures annually; an average of 1 fracture every 3 seconds (International Osteoporosis Foundation, 2017). Data suggest that as much as 10 percent of fractures fail to heal properly (R.T. Franceschi, 2005). In this context, the critical need for safe, effective restorative therapies to stimulate bone regeneration is self-evident.
The primary goal for this section of the curriculum unit is for students to engage with and reinforce the fundamental idea that health ailments are just changes in functional capability owing to changes in structure. Students will stress the idea that solutions for bodily maladies arise from restoring normal structure and/or mollifying the effects of abnormal structure; once again structure-function-dysfunction. In analyzing each of the restorative therapies to be presented in this section and comparing between them, students will draw on their understanding of normal bone development and remodeling (presented in part one). The therapies are presented as real-world concrete applications of content students are mastering using a simple, but logical and coherent framework. The details (mechanisms of action, procedures, and the like) are important, but in teaching this curriculum unit, the points of emphasis will be on the overarching idea of how the big ideas fit together. Additionally, the particular methods presented in the subsections to follow are not meant to represent an exhaustive list of medical interventions in conditions of bone loss or bone damage. Rather, they are compelling examples chosen in furtherance of illustrating the big picture. Other instructors, or indeed the author in subsequent offerings of the course, could very well substitute other interventions while still allowing the curriculum unit to achieve its overall aim.
Bone Grafting
Bone grafting is the go-to procedure for bone reconstruction and fracture healing in cases of surgery, trauma, and degeneration as have been described. (Lu, Chang, Lin, Li, & Hu, 2013) More than 500,000 of these procedures are performed each year in the U.S.; globally the estimate more than doubles. (Greenwald, et al., 2001) Bone grafting is a surgical procedure that replaces missing bone to allow for the appropriate spacing and/or scaffolding required for the biological processes involved in regenerating bones – osteogenesis, osteoconduction, and osteoinduction. Osteogenesis involves the formation of new bone from transplantation of osteocompetent cells (i.e. osteoblasts). Osteoinduction involves formation of new bone from the differentiation and stimulation of undifferentiated mesenchymal stem cells. Osteoconduction involves formation of new bone along a scaffold from the host osteocompetent cells at the recipient site. (Roberts & Rosenbaum, 2012)
There are various types of bone grafts differentiated largely based on the source of the transplanted bone - autologous bone grafts, allogeneic bone grafts, xerographs and synthetic bone substitutes. Each variation has particular risks and benefits associated, as does the general procedure itself. Autologous bone grafts involve harvesting donor bone from sites of non-essential bone (e.g. the iliac crest of the pelvis) in the same individual receiving the graft. Autologous grafts are the gold standard because the transplanted bone possesses all the necessary biological processes. Furthermore, these grafts are by nature immunocompatibile, thus bypassing risks of rejection and disease transmission. Still, there are drawbacks. Autologous grafts may be limited by the amount of bone available from the donor site, donor site morbidity, and the need for bone harvesting procedures (surgical risk at a second site) (Lu, Chang, Lin, Li, & Hu, 2013). Allografts are obtained from individuals of the same species, typically cadavers, thus alleviating donor site concerns associated with autographs. The tradeoff is an increased risk of infection and disease transmission. Alloplastic (synthetic) graft alternatives lack bioactive properties and thus can only serve in an osteoconductive capacity (Lu, Chang, Lin, Li, & Hu, 2013).
Augmentation with protein growth factors
Of note in the previous section is that the interventions described were relatively simple in terms of the overall scheme; surgeons merely sought to enhance endogenous regeneration processes by inserting structures that approximate the normal state. Even when the interventions appear to increase in complexity, there is an adherence to such a principle. This is evident with restorative therapies involving growth factors. The application of biological signaling molecules (growth factors in this case) seek to stimulate the host’s natural healing responses and regenerative repair capabilities while avoiding the disadvantages of the more invasive procedures described in the previous section.
An apt example for this sort of intervention is application of born morphogenic proteins (BMPs), the most well-studied growth factors in bone regeneration. The BMPs are a large group of structurally related proteins that belong to the transforming growth factor-beta (TGF-β) superfamily. By mechanisms of action involving signaling the chemotaxis, proliferation, and differentiation of osteoprogenitor cells, and ultimately, the induction of bone formation by these cells, multiple BMPs have been shown to be closely involved in the processes of bone formation and regeneration (Nauth, Ristevski, Li, & Schemitsch, 2011). For several decades researchers have been able to use restriction enzymes to insert genes from one organism (e.g. human BMP) into the genome of another (e.g. a Escherichia coli plasmid) – recombinant DNA. In such an example, the bacterial mechanism could be highjacked to produce a human protein. By these recombinant methods, two BMPs are widely available and approved for limited clinical use; recombinant human (rh)BMP-2 and rhBMP-7 (Nauth, Ristevski, Li, & Schemitsch, 2011). The effectiveness of these bacteria-produced human protein products in promoting bone regeneration has been evaluated in clinical studies of non-union, bone defects, open tibial fractures, and spinal fusion, with some trials showing regeneration results comparable to auto graft procedures. One such study (Friedlaender, et al., 2001) examined tibial non-union patients randomized in two groups: one group received autologous bone grafts, the other received rhBMP-7. All other aspects of their restorative therapy were identical. The data from the Friedlaender group showed the two groups to be comparable in ability to support weight (81% of the recombinant cohort versus 85% of the graft cohort) and radiographic evidence of union (75% versus 84% respectively). The rate of infection in the recombinant cohort was a third of that of the graft group (4.9 % versus 13%). Furthermore, by definition none of the recombinant cohort, experienced the long-term persistent donor site pain reported by 20% of the graft cohort. In sum, the Friedlaender data showed equivalent results for BMP-based therapy when compared to autologous grafting, while avoiding the morbidity of graft harvest (Friedlaender, et al., 2001).
While the advantages of protein growth factors are significant and promising, there are limitations that make their use in many cases less than ideal. Human proteins produced by E. coli bacterial machinery are not modified in the same way as by human cells. Recombinant proteins of this kind lack the post-translational glycosylation (modifications made after initial protein assemble for proper folding, stability or other purposes) that is present in the endogenous protein (Lo, Ashe, Kan, & Laurencin, 2012). Consequently, these recombinant growth factors tend to be less stable and less biologically active. In part due to the limited bioactivity, treatments of this kind require high doses (which are given by injection) (Southwood, Acvs, Frisbie, Kawcak, & Wayne Mcilwraith, 2004). It is speculated that E. coli’s central role in recombinant growth factor production also increases the possibility of contamination from trace amounts of biologically active impurities. Owing to their relative size (compared to non-protein substances) these protein growth factors are more likely to induce unwanted immune responses from the host. Beyond these drawbacks of the recombinant protein product, the cost of the manufacturing process itself is significant (Southwood, Acvs, Frisbie, Kawcak, & Wayne Mcilwraith, 2004). The burden of high cost to produce a large molecule with some instability and the potential to trigger an immune response currently limit the utility of this restorative therapy .
Augmentation with small molecules
In the previous subsection, the limitations on the effectiveness of the interventions was in large part associated with the size of the protein growth factors. Comparatively, even small proteins are big in terms of biological molecules. In this context then, a substance with the bone-promoting properties of growth factors but without the concomitant heft would be of great interest to researchers; possibly delivering the benefits without many of the drawbacks. And indeed, research does seem to show that such molecules counteract each of the previously described drawbacks. With regard to immunogenicity, small molecules can be small enough to avoid triggering an immune response. Considering stability, whereas proteins generally need to be stable and maintain particular orientations to retain bioactivity, small molecules do not require the similar levels of structural integrity and rigidity (Lo, Ashe, Kan, & Laurencin, 2012). Not insignificantly, large protein growth factors tend to require high doses and are generally administered via injections (editorial note: multiple injections, probably with big needles – because for those who are averse to needles, is there really a such thing as a “not big” needle?), whereas smaller molecules are more likely to be administered orally (Lo, Ashe, Kan, & Laurencin, 2012). And lastly, compared to the process of manufacturing recombinant proteins, many small molecule drugs are inexpensive organic compounds (Lo, Ashe, Kan, & Laurencin, 2012).
Of course, these molecules also have their own drawbacks, namely non-specificity and shorter half-lives. Even still, the discovery of small molecules with osteoblastic differentiation capability is of great interest to researchers in this field. Molecules of this class include but are certainly not limited to: purmorphamine, statins, phenamil, rapamycin, and icariin among several others. (Lo, Ashe, Kan, & Laurencin, 2012) Any of these would probably be appropriate as the example for this subsection of the curriculum unit, but an even more intriguing example takes the idea to an extreme of sorts; even smaller molecules – ions. Several ions - boron (B3+), calcium (Ca2+), cobalt (Co2+), copper(II) (Cu2+), fluoride (F-), lithium (Li+), magnesium (Mg2+), niobium (Nb5+), phosphate (PO43-), silicate (Si4-), silver (Ag+), strontium (Sr2+), vanadium (V5+), and zinc (Zn2+) - have been shown to be capable of inducing osteoblast precursor differentiation through growth factor signaling pathways, or to stimulate other processes in support of bone tissue growth (Lo, Ashe, Kan, & Laurencin, 2012). The size-related benefits (lower cost, stability, efficacy at low concentrations, etc.) highlighted previously pertain here as well. A further distinction is that these molecules are even simpler than the other molecules. Non-specificity and shortened half-lives are still drawbacks, and the challenge for research is to develop techniques for delivery-appropriate dosages in targeted locations over time. Still, such challenges should be more manageable with these molecules in comparison to larger, more unwieldy substances.
Augmentation by Gene Therapy
The progression of the preceding subsections moves toward interventions on smaller and smaller scales. Though this progression lends a sort of logical flow to the curriculum unit, it is certainly not intended to suggest that simply reducing the physical scale of the intervention inevitably makes for an improved or even preferred outcome. It is the precision of the intervention that matters, not necessarily the size of mediating structures involved. The interventions presented in this section will provide examples of targeted precision by another means; namely, gene therapy.
One of the significant limitations of protein growth factor mediated bone regeneration is the reduced stability and bioactivity of the recombinant protein due to a lack of glycosylation resulting from the use of E. coli cellular machinery. These less-stable, less-active recombinant proteins are then more likely to degrade too soon and less effectively promote bone formation. Gene therapeutic approaches are able to avoid such challenges because rather than transferring a large E. coli-made protein, vectors are used to transfer the genes. In this way, the hosts cellular machinery is used to produce a protein the undergoes the appropriate post-translational processing (e.g. glycosylation). Such a protein, with improved stability and bioactivity will more effectively function to help to regenerate bone.
After having examined these previous established and some novel approaches for augmenting bone regeneration, students will conclude this part of the unit by considering interventions that may be on the horizon. Perhaps no scientific development holds more potential for advancing medical intervention than gene editing approaches using CRISPR-based technology. The advent of CRISPR technology has had wide reaching impact in many areas of medical science and in the wider public imagination. Researchers are beginning to view bone regeneration as one such area of potential gene-based interventions. Here, students will be introduced to the basic mechanisms of the CRISPR machinery and be presented with non-bone examples of CRISPR applications, so that they can consider potential bone-regeneration applications.
So, what exactly is CRISPR? In short, CRISPR technology is a simple yet powerful tool that allows for editing of an organism’s genome. CRISPR (or Clustered Regularly Interspaced Short Palindromic Repeats) is an evolved defense mechanism that naturally occurs in some species of bacteria (Cong & Zhang, 2015). CRISPR provides a defense for cells by readily identifying invading viruses based on fragments of viral DNA from previous attacks that have been incorporated in a CRISPR array in the bacterial chromosome. Together with the bacterial protein Cas9, the system is able to cut and remove parts of the virus in order to deactivate it. Researchers have taken advantage of this bacterial defense mechanism and designed a CRISPR-Cas9 system that can target and edit DNA at precise locations [See figure 2]. A cell is transfected with a DNA plasmid that expresses both the Cas9 protein and a sequence of guide RNA (gRNA), which matches and binds to the gene of interest. The Cas9 protein then identifies the corresponding sequence on the host cell’s genome and initiates a double stranded cut in the DNA. The double strand cuts activate a cascade of signals whose ultimate goal is to repair the cuts, which will occur in one of two ways (3a and 3b in figure 2). By one method, non-homologous end joining (NHEJ), the targeted gene is removed because cut ends of DNA are joined together without the gene between them. In the other method, homology directed repair (HDR), a replacement gene whose flanking sequences were designed to match the cut sites is inserted in place of the original gene (Cong & Zhang, 2015).
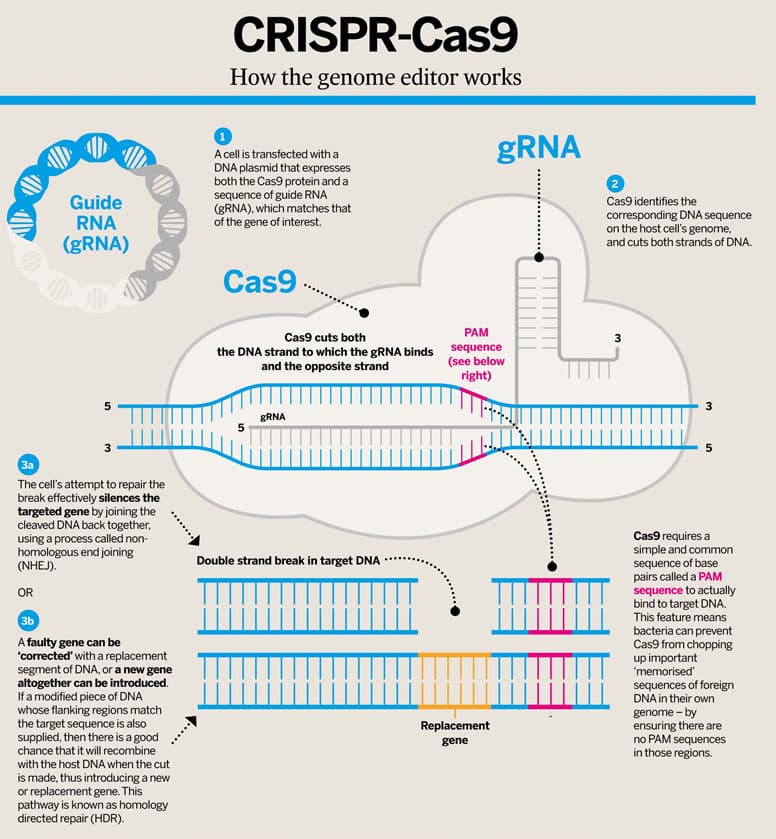
Figure 2- CRISPR-Cas9 Mechanisms
With such a tool, the gene therapeutic possibilities are vast. One such example of the applications of CRISPR comes from the Parker Institute for Cancer Immunotherapy. Using the CRISPR-Cas9 system the researchers designed an elegant approach to help the body fight cancer. In this novel approach, researchers remove T cells from cancer patients and perform three CRISPR edits on them. One edit inserts a gene for a protein engineered to detect cancer cells and instructs the T cells to target them. Another edit deletes a gene for an endogenous T cell protein, PD-1, that normally inhibits T cell response (a check point of sorts). The last edit inhibits cancer cells to disable T cells because it removes a protein that identifies the T cells as immune cells. The modified T cells are then returned to the patient (Reardon, 2016) (Engineering T-Cells to Fight Cancer | Parker Institute for Cancer Immunotherapy, 2017). The simplicity and specificity of the CRISPR allows these scientists to construct a two-pronged approach that both enhances the ability to attack cancer cells and inhibits the ability of the cancer cells to attack the T cells. Early results of the study are promising, and the approach has already been approved for human clinical trials.
Presently the technology is so new, that little research about the possible bone regeneration gene targets of CRISPR technology have been published. This situation presents a unique opportunity to challenge students to develop their own gene therapeutic CRISPR-mediated schemes that could be predicted to have positive outcomes. The big picture construction of CRISPR technology (it’s as easy as removing a typo from a sentence and re-writing it correctly) can be easily understood by students and as such there should be some overlap in schemes suggested by students and the work that researchers are pursuing. For example, following the T cell example in the previous paragraph, a student with even a basic understanding of bone regeneration and of CRISPR technology could think some version of the following: “my goal is to have bone heal and regenerate itself more quickly and more efficiently. What if I delete genes that slow down repair and spread genes that speed up repair?”
In a recent abstract for a research proposal, Dr. Adalberto Luiz Rosa wrote that he has selected genes involved in either promotion or inhibition of osteogenesis as targets in a CRISPR-based approach to augmenting bone regeneration. His group intends to use CRISPR to overexpress BMP-9 which initiates cartilage and bone formation and also knock out periodontal ligament-associated protein-1 (PLAP-1) which acts as an inhibitory factor for osteogenic differentiation and bone formation. Rosa expects data from this research will lead to viable alternative therapies for the repair of bone defects and non-union fractures (Rosa, 2017). The simplicity of the approach and its relation to the very questions students were expected to have generated earlier, allows this research proposal to serve as another point of emphasis for connection between basic principles explored in the classroom and cutting-edge medical interventions being developed in the real world.
Part 3 – Scientific Judgement: Risks, Consequences and Ethical Considerations of research.
CRISPR technology and its potential have captured both the scientific and popular imaginations. Researchers and laypeople alike are intrigued and absorbed by its seemingly endless capacity to provide a remedy for every condition from cancers to male pattern baldness. In this exuberant context and the drive to push the possibilities forward, there is the argument that perhaps insufficient consideration is given to risks and dilemmas arising from the new and advancing capabilities. In this part of curriculum unit students will consider risks and consequences associated with gene-based therapies, and then grapple with some of the ethical conundrums newly emphasized in the development of this technology.
The pace at which CRISPR technology has spread is remarkable. In little more than a half-decade since its emergence, it continues to gain momentum in research and laboratory settings because it is considered precise, fast, cheap, and easy to use. It is also for these very reasons that is necessary to pause and soberly assess the risks. In a 2017 review published in the Yale Journal of Biology and Medicine, Cribbs and Perera summarized some of the most compelling potential risks associated with CRISPR-Cas9 gene editing technology. Cribbs and Perera divided potential risk into two groups; technical concerns and social concerns. Within the technical group were further subdivision for off-target insertions and deletions (Indels), random integration of vector, and toxicity. Across all categories risks were considered for germline editing, ex vivo delivery (cell transplants), and in vivo delivery (tissues and organs) (Cribbs & Perera, 2017). The results are summarized in table 1.

One take-away apparent from the table is the relative high risk associated with potential inadvertent insertions and deletions (indels). That is the example to be focused on in this part of the unit. In downplaying the possibility of unintended and unforeseen consequences, researchers have lauded the specificity of CRISPR targeting; CRISPR is specific because the gRNA pairs with a unique sequence to initiate the mechanism. There has been a general acceptance of these conclusions about specificity because to this point exploration of Cas9-induced genetic alterations has been limited to the immediate vicinity of the target site and distal off-target sequences (Kosicki, Tomberg, & Bradley, 2018). However, in a July 2018 paper in Nature, researchers from the Wellcome Sanger Institute call for more scrutiny and caution in using CRISPR technologies, citing more unintended mutagenic events associated with CRISPR than previously reported. Whereas the majority of DNA repair outcomes in CRISPR were thought to be indels of less than 20bp, they report large deletions of thousands of base pairs and other complex rearrangements (Kosicki, Tomberg, & Bradley, 2018). The WSI researchers speculated that a substantial portion of genotypes resulting from Cas9 repair may have been missed for several reasons (e.g. the research often used cancer cells with abnormal DNA repair mechanism thus making it problematic to extrapolate to normal cells – also the scope of the assessment was limited because studies relied on amplification of short regions around the target and potential off-target sites.) A significant concern is that, in actively reproducing cells, these newly reported deletions and rearrangements can have significant pathogenic consequences (e.g. cancer) (Cribbs & Perera, 2017).
These results emphasize the need for deliberate caution as appropriate when pushing forward with CRISPR technology. Beyond the obvious concern for individual patient outcomes, it is also important to note that with regard to research in medical science, unintended consequences that lead to bad individual outcomes, can resonate across the field and set back all manner of promising research (especially when they play in to existing public skepticism and unease).
Beyond questions of efficacy and safety, gene therapies are often controversial due to associated moral and ethical questions. As with other scientific developments, as the technology advances, questions shift from ‘can we’, to ‘should we’.
By this part of the curriculum unit, students now have a firmer foundation of science on which they can meaningful engage with some of the pertinent ethical questions of the day.
Among other issues, students will consider the following questions presented on the NIH Genetics Home Reference website: How can “good” and “bad” uses of gene therapy be distinguished? Who decides which traits are normal and which constitute a disability or disorder? Will the high costs of gene therapy make it available only to the wealthy? Could the widespread use of gene therapy make society less accepting of people who are different? Should people be allowed to use gene therapy to enhance basic human traits such as height, intelligence, or athletic ability?
Ultimately students should be about to weigh risks and benefits (present day and potential) associated the gene-based therapies (CRISPR in particular) and formulate responses to the question “When do the benefits outweigh the risks?”

Comments: