Content Background
Radiation Fundamentals
Everything is radioactive! Everyone is exposed to radiation! Exposure to radiation is as natural as being under the sun. In fact, the sun is the source of radiant energy. Radiant energy is emitted and transmitted in waves rather than through matter. We know these waves to be a part of the Electromagnetic Spectrum (EMS). (See Figure 1 – Electromagnetic Spectrum.) EMS shows the correlation between wavelength, frequency, and energy. As we move past the visible light spectrum, wavelength, the distance between the crest of each wave, decreases as the frequency, or rate at which the wave passes over time, increases. Using the formula we can calculate the amount of energy associated with each type of wave. Energy can be calculated knowing h = Planck’s constant 6.33 x 1034 Js and f = frequency (Hz). Or, energy can be calculated with the wavelength ( and the speed of light in a vacuum c = 3 x 108 m/s.3
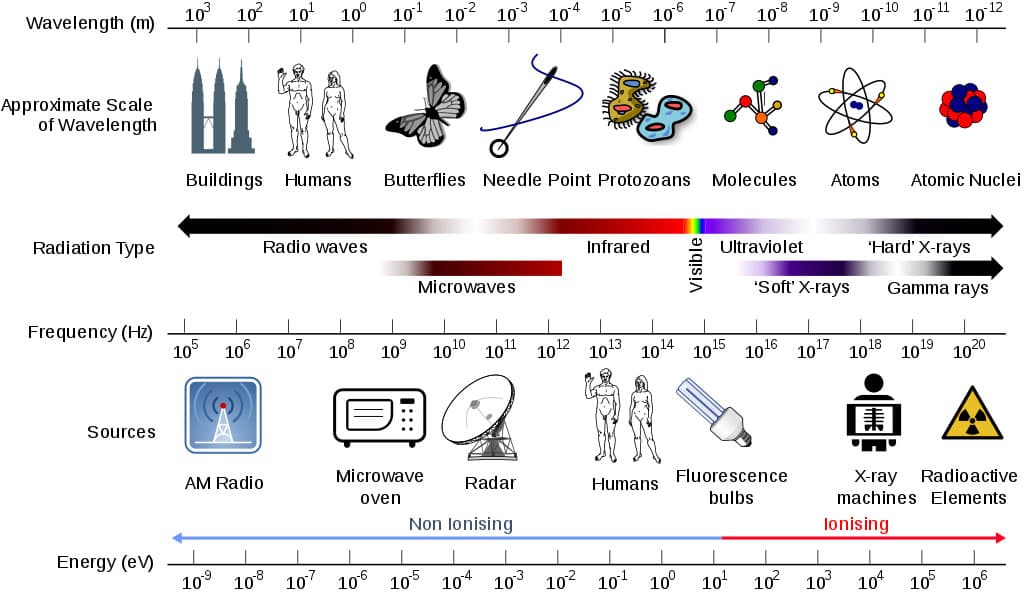
Figure 1. Electromagnetic Spectrum showing wavelengths, frequency, radiation types, and energies associated.4
This unit will primarily focus on ultraviolet radiation, X-rays, and gamma rays which are all types of ionizing radiation whose wavelengths are shorter than 400 nm. To begin understanding why there are some types of radiation that are more harmful than others, a review of atomic particles is necessary. Many students will recall the atom. The atom is a unit of matter. The three atomic particles are positively charged protons, neutral neutrons, and negatively charged electrons. Within the nucleus of an atom are protons and neutrons. Because protons are positively charged, they tend to repel (push) each other away; thus, neutrons hold protons together. Atoms vary in the number of neutrons and protons they have. This variance in neutrons and protons creates different elements. Because atoms are high energy, they at times lose or change the number of protons and neutrons that they have. This change in the atom is known as radioactive decay. For example, uranium loses 2 of its protons to become the lighter thorium with 90 protons. This decay matters because each time an atom decays, it creates radiation.5
There are three types of radiation produced by radioactive decay: alpha, beta, and gamma radiation. Each are created from an atom trying to balance itself out (having enough neutrons to hold the protons together). Unstable nuclei undergo radioactive decay when a nucleus absorbs another particle or loses a particle. This shift in an atom where there are more protons than neutrons or vice versa creates radioactivity. (See Figure 2- Radioactive Decay.) In alpha radiation, clusters of 2 protons and 2 neutrons are expelled from an atom. This particle is completely harmless outside of the human body, but harmful within the body because it could lead to radiation sickness and other radiation related disorders. Beta particles are released when radioactive atoms expel negatively charged electrons. Unlike alpha particles, these are very light and fast moving. The third type, gamma radiation, is like light rays, but different from alpha and beta particles since gamma radiation has no mass and has no charge. Gamma rays are emissions of photons (a particle of light or electromagnetic radiation). Each particle has its own level of harmfulness to the human body which will be discussed later in the unit.
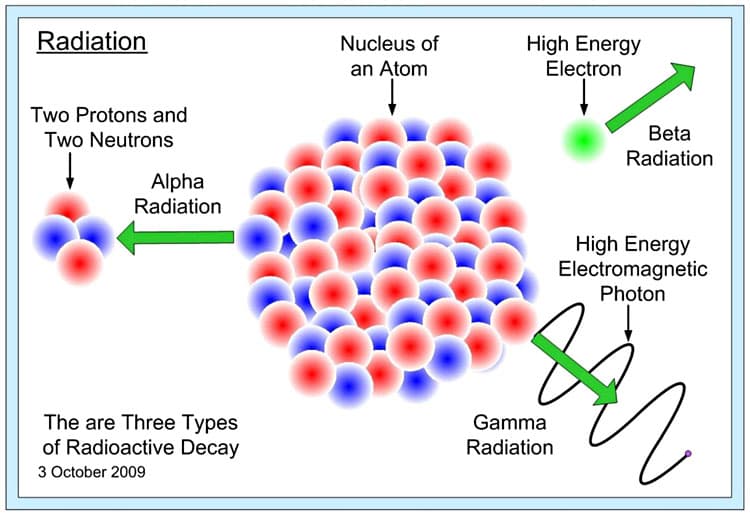
Figure 2. Radioactive decay showing alpha, beta, and gamma radiation.6
When measuring radiation there are four components: Radioactivity, Exposure, Absorbed dose, and Dose equivalent. Radioactivity is the amount of ionizing radiation released during over time. This is measured in curie (Ci). Exposure is the amount traveling through air. This is measured in roentgen (R). Absorbed dose is the amount that is absorbed by a person or an object and it is measured in radiation absorbed dose (rad). Dose equivalent measures the combined absorbed radiation and the effect of the radiation on the human body. This measurement is expressed as roentgen equivalent man (rem) and 100 rem equals 1 Sievert (Sv). Thus 1 R (exposure) = 1 rad (absorbed dose) = 1 rem or 1000 mrem (dose equivalent).7 To put things in perspective, 1 mrem is equivalent to 1 year of living next to a nuclear power plant or 1 flight coast to coast. When discussing exposure to radiation, it would take a very high dose of radiation to cause radiation sickness or death. In fact, it would take about 2,500 mrems to cause radiation sickness. According to an article in MIT Tech News, the limit of acceptable exposure is 3000 mrems for adults, while children under 18 should be limited to under 500 mrems. The average American citizen is exposed to 620 mrem each year, well below the acceptable exposure limit.8
Sources of Radiation
Natural Radiation
We are all radioactive. Atoms in our bodies decay; we eat food, inhale particles, drink water, and interact with other humans and animals who are all emitting radioactive particles. Gale and Lax in Radiation proclaim, “Sleep next to someone and your bedmate will get a dose of Potassium-40 radiation from you” and vice versa.9 According to the U.S. Environmental Protective Agency, background radiation includes radiation from the ground and soil, water, and even other humans.10 Radiation from radon, is particularly prevalent with 37% of all natural radiation sources coming from radon.11 Radon-222 is so prevalent that most people have ingested it or inhaled it from air, water, or soil. Rn-222, in fact, is attributed to 21,000 lung cancer deaths each year.12
Ultraviolet Radiation
One type of radiation that my students are particularly interested in studying is ultraviolet radiation. Beyond the visible spectrum is the ultraviolet spectrum. Wavelengths ranging from 10 to 400 nm include three types of UV (A, B, C). Because of the earth’s atmospheric composition, most UV rays emitted by the sun are absorbed by the ozone layer. UV-C rays never make it past our ozone layer, while UV-B and UV-A rays do. The more harmful of the two are UV-B rays which have the potential of harming DNA structures. Fortunately, only 5% of UV-B strikes the earth’s surface.13 Most of the damage done to people’s skin is caused by UV-B radiation.
UV-B rays cause suntans through the stimulation of melanocytes to produce melanin. The cells associated with melanin production, melanocytes, are not unique to the skin. They are found in the brain, the heart, and mucous membranes of the GI tract. Because melanin does not stay in the skin forever it is passed to keratinocytes, cells with a short life span of 4 to 5 days. UV-B exposure causes damage to the DNA of melanocytes. If the DNA is damaged, it may alter the cell’s ability to regulate division causing uncontrolled cell growth. Uncontrolled cell growth could potentially cause cancers, like melanoma. According to the World Health Organization it estimates 65,000 melanoma related deaths occur each year. Though the best way to protect the skin from UV-B rays is simply to cover the exposed skin with long sleeves, UV protective sunscreen can provide some level of protection if used properly.14 However, sunscreen does not completely block out UV, it merely allows the user to stay exposed longer than not having any protection. The combination of chemicals like zinc oxide reduce penetration of UV rays. A sun protective factor (SPF) label of 20 means the sunscreen can absorb 95% of the harmful UV. Sunblock wearers should still recognize that 5% of UV rays pass the skin which may cause sunburn or premature again over time.
But, how do UV rays damage the skin? First, students need to recall the structure of DNA. DNA is composed of four bases which include combinations of adenine, cytosine, guanine, and thymine. Alone, UV-A cannot damage DNA directly but can cause free radicals which may harm DNA indirectly along with other fats and proteins. UV-B rays on the other hand affect the bonded thymine bases by altering their chemical bonds. Exposure to UV-B creates thymine dimers. When dimers of thymine are formed it creates a kink or bend in the normal structure of DNA which alters the DNA message. This change could lead to mutations, shifts in the DNA code which could lead to improper coding, cancer, or cell death.15
Besides creating thymine dimers, DNA bonds can be altered by exposure to higher energy UV. Referring to Figure 3, bonds forming the DNA bases are made of bonds between carbon (C), nitrogen (N), oxygen (O), and hydrogen (H). Respectively, the bond between H and O has 467 kJ of energy, while the bond between N and H has 391 kJ of energy. (See Table 1.) According to the electromagnetic spectrum, UV-C rays can have energy values greater than 427 kJ which would be strong enough to break the bonds in adenine, thymine, guanine, and cytosine. Even if a person were only exposed to UV-A rays, like the kind that penetrates the skin, it has enough energy (299-373 kJ) to break C-C bonds (347 kJ), C-N bonds (305 KJ), C-O bonds (358 kJ). As the graphic (Figure 3) shows many of the bonds holding together the DNA structure can be damaged by UV radiation.16
The bottom line is simple, UV radiation’s energy has the potential to affect DNA because the chemical bonds holding the bases, to create the DNA ladder are not that strong. This of course is essential to DNA’s ability to replicate but it is also the reason why DNA is susceptible to damage from high energy radiation. Fortunately, the skin’s DNA has a variety of methods to protect itself from damaging UV radiation.
Despite UV’s reputation to cause sunburns, break collagen fibers that cause premature aging, and damage DNA, UV rays can be beneficial. For example, UV-B synthesizes vitamin D which is necessary for bone remodeling. It also prevents Rickets which is a deficiency in vitamin D causing bones to soften. Another benefit of UV radiation occurs in psoriasis patients whose skin produces silvery, flaky, scaly patches. Treatments with a dye called psoralen and UV-A rays kills the DNA of cells with psoriasis allowing healthier skin cells to proliferate.17
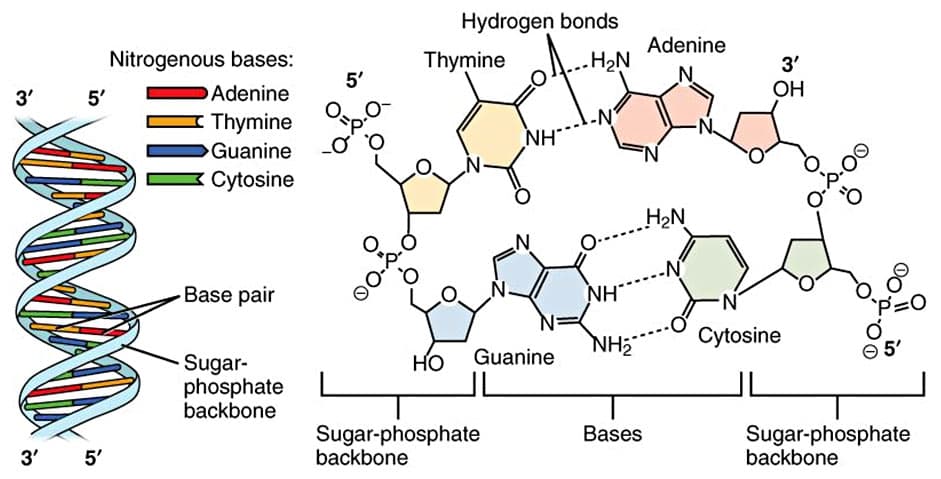
Figure 3. DNA Structure showing chemical bonds between DNA base pairs.18
|
Type of Bonds in DNA |
Energy Content in kJ/mol |
Broken or Damaged by |
|
Oxygen - Hydrogen |
467 |
UV-C rays (>427 kJ/mol) |
|
Carbon - Hydrogen |
413 |
UV-B rays (373-427 kJ/mol) |
|
Nitrogen - Hydrogen |
391 |
UV-B rays (373-427 kJ/mol) |
|
Carbon - Oxygen |
358 (single bond) |
UV-A rays (299-373 kJ/mol) |
|
Carbon - Nitrogen |
305 (single bond) |
UV-A rays (299-373 kJ/mol) |
|
Carbon - Carbon |
347 (single bond) |
UV-A rays (299-373 kJ/mol) |
Table 1. Bond and UV energies,19 information compiled and modified from Clutch Prep Chemistry and Gary Brudvig’s YNI Energy Science seminar 2019.
Man-Made Radiation
My students may be cognizant that sunblock is necessary to maintain healthy skin. But, sunblock is not a priority in their lives. They are more concerned about one thing – power. Power to fuel and charge their cellular devices. Each day, students enter my classroom and inevitably one of them will ask if they can charge their phones, not being remotely concerned where the electricity to power their phones comes from. Granted, living in California with its 8 months of sunny weather means we have plenty of power from the solar sources, but what they do not realize is that 9% of California’s power consumption comes from nuclear energy.20
Nuclear Energy
How is nuclear energy generated? Nuclear energy is generated during nuclear fission. In nuclear fission, neutrons bombard the element uranium (U), which is a heavy metal that is mined. The crashing of a neutron into the nucleus of a uranium atom causes a splitting of the atom, a breaking of the attractive force between protons and neutrons in the nucleus, which in turn releases energy. This energy, as heat, warms up the surrounding water where uranium rods are submerged. The water warms creating steam, and powers turbines to produce electricity.21 The fuel used in nuclear reactors comes from enriching uranium. (See Figure 4.) Uranium as an element is mixed with other minerals, sulfuric acid, and oxygen to form uranium oxide. In powder form, this material is not very radioactive and needs further processing to enrich the U-235 relative to the more abundant U-238. The mixture of U-238 and U-235 needs to be mixed with fluorine to obtain uranium hexafluoride (UF6), which is a gas. The once the powdery uranium oxide material is converted to a gas, UF6 is centrifuged thousands of times to separate U-238 from U-235 the desired type of uranium to make uranium pellets. Once the enrichment process produces a lot of U-235, the gas is combined with calcium in order to create uranium pellets. Pellets are used as fuel in reactors and the spent U-235 is turned into plutonium (Pu). All throughout this production of nuclear fuel, radioactive rays and particles are created and released when atoms break apart.22
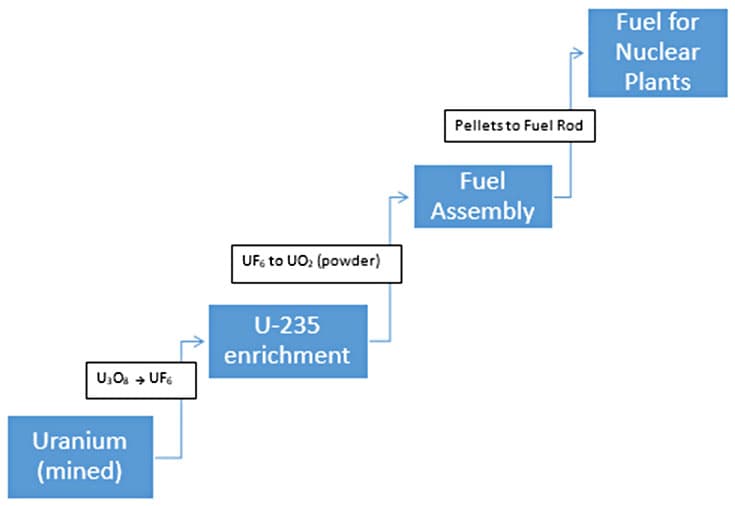
Figure 4. Processes involved in production of nuclear fuel.
Despite potential for supplying and providing the world with over 2500 TWh of electricity, nuclear energy is disfavored in many countries. There are very few reactors building built, with France as the only country that is committed to increasing its nuclear use.23 The fear of course stems from the disasters at Chernobyl in 1986 and Fukishima Daiichi in 2011. The general population does not see how safe nuclear reactors are since most media only publicize instances when reactors fail. Granted, the high energy contained in nuclear reactors and its potential to cause radiation sickness, radiation related cancers in some, and birth defects in pregnant mothers are alarming. But, we also must balance nuclear disaster image with those of people being hurt during natural gas explosions, cancers related to coal mining and fracking, and exposure to toxic waste materials during the production of supposed clean energy sources. As with most new development, there is a consequence that must be faced or a cost that must be paid. With regards to nuclear energy, the benefits might outweigh the few instances in which people were injured, became ill, or killed.
Radiation in Medicine
Nuclear fuel and nuclear weapons are not the only uses of radioactive materials. Today, the field of medicine has expanded the use of radioactive and nuclear materials to diagnose and treat patients. This unit will discuss the use of X-rays, a high energy electromagnetic wave, and radionuclides (radioisotopes) in medicine.
Most people will at some point be exposed to radiation in a dental or medical office in the form of X-rays. X-rays are high energy photons (light energy) that are invisible to humans since they do not fall within the visible light spectrum of 380 to 740 nanometers. Because X-rays are high energy, they can penetrate the skin but cannot penetrate bones. The shadow that is cast by bones creates an image on an X-ray film. This shadow does not occur when soft tissue like skin and muscles are exposed to X-rays. Bones appear white because of a mineral called hydroxyapatite. Hydroxyapatite combines calcium phosphate and calcium carbonate (Ca5(PO4)3(OH).24
The Nobel Prize of 1979 was awarded to Godfrey Hounsfield and Allan Cormack who further developed the use of X-ray technology that became the computer axial tomography (CAT or CT scan). Through a series of 175 to 200 X-ray scans, a full three-dimensional view of a patient’s body can be visualized, giving physicians a powerful diagnostic tool. Similar to mammograms, the benefits of exposing the body to far greater ionizing radiation outweighs the bad and “the decision to recommend any medical screening procedure…is based on a delicate balance of estimating potential benefit and risk.”25 The benefit of exposing a patient to higher dose of radiation in CT scans is that CT’s produce a highly detailed image of a small cross section of the body. When combining the cross sections, doctors can get a more comprehensive view of the body or a specific organ system to assist in their diagnoses.26
Though X-rays and CT scans have allowed doctors to diagnose medical problems for over 100 years, more inventive use of radiation has accelerated our ability to detect and diagnose disease with the use of nuclear medicine. Though the term, nuclear, brings pause and hesitation to patients, radioactive injections and radiotherapy are important tools in today’s medicine. Radiotracers, allows doctors to diagnose heart disease, gastrointestinal issues, endocrine dysfunction, and even neurological abnormalities. For example, if a patient noticed they were losing weight but had not changed their diet or exercise, a doctor might want to confirm if the patient has hyperthyroidism. This condition causes the thyroid gland to over produce its hormone thyroxine which might be one cause of unintentional weight loss.27
According to Gale and Lax in Radiation, “[Radiation Therapy] is like a nuclear weapon where the antibody is the targeting missile & radionuclide is the nuclear warhead.”28 Radiation Therapy (RT) is given to patients in several small doses over the course of days or weeks. This mode of treatment allows normal cells to recover from the high dose of radiation while killing off the targeted cancerous cells. RT is being used to eradicate cancerous cells, prevent reoccurrence of cancer cells, and reduce the size of a cancerous mass. Like other therapies, the effectivity and damage are dependent on an organs’ sensitivity to radiation. Organs like bone marrow, gastrointestinal tract, and skin are highly susceptible and sensitive whereas the brain, kidney tolerate higher levels of radiation.29
The world is filled with harmful substances. Radiation is just one of many substances that is potentially harmful and could damage the human body. It is important to not let one source inform our decisions about radiation. The information and exaggerations we hear in the news must be mitigated with fact based on science and research. Admittedly, nuclear energy can pose hazards if nuclear reactors are not well managed, but, most people will never encounter radiation from a nuclear facility. It is more likely that radiation exposure will come medical sources, the food that we eat, and the sun. Since we cannot avoid radiation nor its benefits, each of us can be proactive by monitoring our own radiation exposure levels. Websites like the Environmental Protection Agency offer radiation calculators which allow users to input multiple variables like elevation, proximity to nuclear facilities, occupation, and medical procedures to calculate radiation exposure levels per year. In doing so, the public can inform themselves about their actions that increase or decrease their exposure levels. More importantly, the public may come to realize the truth, that nuclear power plants and radiation are not our enemy. We can easily implement methods to control our exposure to avoid radiation sickness. There is far too much good that radiation has allowed humans which simply outweighs the costs.

Comments: