Content Objectives: Energy Transitions and their Consequences
To be able “to think with energy” connects ideas across time and scale, from the atomic to the human, from a classroom to the entire planet.1 All of life is an on-going struggle to capture energy from the environment. Our most memorable dinners with friends and family would not exist without the chemistry of photosynthesis. The world’s first military-industrial complex was largely wind powered,2 and a modern kingdom can be lost for want of a barrel of oil.3 These moments were part of the five energy “transitions” explored in this unit, times when new ways of accessing or using energy changed history in profound and unintended ways.
Although the following five events are far from an exhaustive record of energy innovations, they represent a new or significant improvement to how living things directed energy flow in their environment with clear consequences for each change. The oldest event is the evolution of photosynthetic organisms that produce oxygen, ~2.7 billion years ago. The second, much more recent innovation, was the application of windmills to sawing wood in 1596 C.E. Following the wind revolution was James Watt’s steam engine patent in 1769 that significantly impacted coal consumption. In 1832, Michael Faraday’s electromagnetic induction of continuous current set a required precedent for electrifying “the modern world.” One of the key energy decisions of the 20th century was Winston Churchill transitioning the British Navy from coal to oil powered ships in 1911.
The science and mathematics behind these transitions and their unfolding historical legacy emphasize knowledge as a life-changing and world-changing power. This requires its own mental transition. In a hypothetical polling of ninth grade environmental scientists, one could ask, “What is the most important power for changing your life and the world?” I suspect a majority would identify “money.” This is a compelling question for almost everyone in public education, students, teachers, families, “Why do some people enjoy the power of wealth while others suffer for its absence?”
The relationship of knowledge, energy, and wealth is one of unequal power distribution. Although income inequality has always been large, its increase from 1820 and 1910 can be largely explained by the rise of Western colonial empires,4 whose markets and technologies were fueled largely by application of wind and coal. While there were different forms of colonialism, some of which left industrial infrastructure that promoted long-run investments in education and economic development,5 the goal of extractive capitalism, “was to transfer as much of the resources of the colony to the colonizer,” creating a legacy of exploitative institutions, reduced educational opportunities and resource wealth, and a further divide in capital investment of knowledge and industrial production.6
In 2019, the average U.S. resident consumed nearly eighty thousand kilowatt hours [of electricity] a year; those in Germany and France roughly forty thousand; those in Chad, Niger, Mali, or South Sudan less than one thousand.7
For the 2.6 billion people in 2021 who relied on biomass cooking fuels, the resulting air pollution contributes to premature death from lung cancer, heart disease, and strokes.8 Modern hospitals need reliable access to electricity. Home electricity helps provide clean, running water and refrigerated food. It powers phones and internet access, helps students study at night. Banks and factories rely on electricity to “expand economic opportunity.”9 Historic access to cheap, abundant energy, namely fossil fuels, has reshaped agriculture, transportation, communication, manufacturing, and even politics, leaving others deprived of this “mainspring of modern material civilization.”10 As we face an existential climate crisis caused by burning fossil fuels, it is these people who are most vulnerable to the natural and economic hazards that will result. The history of energy is at the heart of our future.
Energy Basics
To better talk about history and future, we need some basic vocabulary. Like matter, energy is a fundamental property of the universe. It manifests as heat, light, or the ability to do work.11 It can be quantified and measured. It cannot be created or destroyed. Together, those two features combine in the “law of conservation of energy.” There is a certain amount of energy that exists, and it can be transformed between different forms, but the amount does not change.
The different forms of energy can be divided into two major categories, kinetic and potential. When energy is moving, it is called kinetic. Examples include wind and mechanical energy, where air or objects are in motion. Electrical energy is the result of moving electrons. Sound energy is a wave of colliding particles. This category also includes forms that propagate in the electromagnetic spectrum, like light or thermal energy. The other category of energy is not in motion, but has the potential to become motion energy. These include gravitational, chemical, and elastic energy. Most systems can be described as having some combination of these two forms, such as a battery in active use moving electrons through a circuit but still maintaining potential energy in its electrically charged chemicals.
In order to measure the amount of energy in that battery, physicists might try to think about the battery as a “closed system.” They would make a theoretical distinction isolating the battery and its circuit from any other input or loss of energy. However, as the battery converts chemical potential energy to electrical energy moving through the circuit, the battery “wears out,” and it appears that the total energy of the closed system is being reduced. But if the system is closed, where does the energy go?
The second law of thermodynamics states that heat, a measure of thermal energy, will always move from a higher temperature to a lower temperature resulting in equilibrium. This can be restated in terms of “entropy” or how much energy in a system is not available to do work.12 A highly organized system with lots of energy available for work will always move towards one of increasingly chaotic entropy that is less and less useful for work. This inevitable “heat loss” is one of the key struggles of energy conversion, and even has its own definition in the International System of Units (SI) for measuring energy.

One joule, J, is equal to the thermal energy lost when one ampere of electric current, A, passes through a one ohm resistance, Ω , for one second, s.13 For the battery in the closed system above, the energy appears to decrease because the electricity in the circuit experiences resistance, changing to thermal energy and becoming part of the ambient environment which can no longer be directed for work.
How we measure that energy depends on what form is being observed. Joules were intentionally chosen to represent both capacity for electrical and mechanical work.14 When measuring the energy of a battery, one joule is equal to the electricity needed to run a one Watt device for one second.15
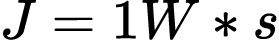
When pushing the battery across a table, one joule is the energy needed to accelerate a one kilogram mass at one meter per second squared over one meter.16
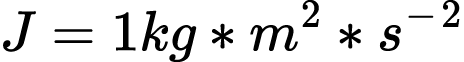
In chemical energy, we measure at how strongly the atoms are bonded together, which can be expressed in kilojoules per mole (kJ/mol). The amount of energy in one photon of light depends on its color but is on the scale of 10-19 joules.17
Converting energy between these different forms is the foundation of life. While biologists often point to the universality of DNA to indicate a common evolutionary ancestor, the drive to obtain energy is also universal.18 It is theorized that early bacteria were also early chemists, moving electrons in hydrogen or hydrogen sulfide gas to create a charge gradient that is more negative on one side and more positive on the other; as the difference is discharged, it creates a “flow” that spins a tiny protein motor for synthesizing adenosine triphosphate (ATP), “the energy currency of the cell.”19 Early cyanobacteria developed a process where sunlight was used to move electrons, transforming light into chemical energy and eventually working together with non-photosynthetic cells to become the chloroplasts of modern plants.20 Herbivores developed mutualisms with gut bacteria to get energy from digesting those plants. Predators adapted sharper teeth and claws to better catch and eat the herbivores.
Formal analysis of “energetics” at the scale of ecosystems became more widespread after 1953 with the publication of The Fundamentals of Ecology by Eugene and Howard Odom.21 Based in part on earlier quantification of predator-prey interactions by Lotka and Volterra in the 1910s, scientists now had an accounting style mathematics for measuring the energy of “biomass” and how efficiently it moved through different “trophic levels.”22 Amazingly, the energy that has fueled human capacity to understand and communicate these processes, much to the chagrin of students who will be tested on them, can be traced to a very old nuclear fusion reaction ninety-three million miles away.
Light Energy and Photosynthesis
Light is a form of electromagnetic radiation that functions as both a wave and a particle.23 Measured in discrete packets of energy called “photons,” the amount of energy is proportional to its frequency, measured in Hertz (Hz). The faster the wave is moving up and down, the more energy it contains. The inverse of that measurement is wavelength,, and in light, the unit is nanometers (nm). If something is moving up and down very quickly, there is not a lot of space between the high point of each wave. The distance between these “peaks” is its wavelength. When a particle is quickly moving up and down, its frequency is high, but the distance between its waves is very small.
This is what produces the spectrum of visible colors. Red light carries the least amount of energy in the visible spectrum and has the lowest frequency. Violet light has the highest frequency and is the most energetic of the visible spectrum. This becomes especially important because high frequency, high energy particles can be dangerous for humans, including “ultraviolet” light, which is just beyond the visible range for humans, and can cause mutations in DNA that lead to skin cancer. Although the term “light” most commonly refers to the visible spectrum that humans can see, it also includes other forms of electromagnetic radiation like gamma rays and x-rays, as well as farther traveling but less energetic forms like Wi-fi and radiowaves. Because of the inverse relationship between energy and wavelength, more dangerous forms of light like gamma rays are not able to travel long distances through matter, while radio waves, whose wavelengths are measured in meters, are so low energy as to be relatively harmless.24

Figure 1. Diagram showing wavelengths of light25
Sunlight contains all the different wavelengths of light and appears white to humans as a result. As the light travels through space, its energy is either reflected or absorbed, depending on what it hits. If all the energy is absorbed, a human would perceive the object as “black.” No photons are being emitted with a particular color frequency. If all the energy was absorbed except the red spectrum, which was reflected off the object, the red light would keep moving until being absorbed by the cone cells in our eye, sending an electrical signal to the brain indicating “red.”
This is why most plants are green. They reflect back the photons our brains register as “green” while absorbing energy from the red and blue-violet spectrum of light. This is what powers photosynthesis. Although it is not the only pathway that early life used to capture energy, it is one of the oldest ways of directing energy from the environment. Theorized to be first developed by bacteria 3.2-3.5 billion years ago, this complex process absorbs light energy from the sun to convert water (H2O) and carbon dioxide (CO2) into energy rich sugars for the organism in the ratio below.26
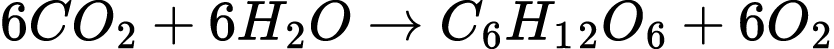
To change low energy reactants to high energy products, photosynthetic organisms must first absorb the light using a combination of pigments, including chlorophylls and carotenoids, such as beta-carotene. Organized in structures called “light harvesting antenna,” they function like a satellite dish, collecting and focusing more energy than single molecules would be able to accomplish on their own.27 This energy is directed to a photochemistry reaction center where it is used to “excite” a molecule into transferring an electron, creating a pair of charged ions.28 The pair is unstable, however, because the electron could transfer back and the energy would be lost as heat instead of useful work.29 The separation happens in less than a nanosecond and in optimum conditions is nearly 100% efficient in converting light energy from photons into chemical products.30
To store the energy for the next phase, the negative and positive charges must be further physically separated. How this is accomplished varies between different photosystems, but most organisms that produce oxygen during photosynthesis transfer electrons such that energy is stored in ATP molecules and nicotinamide adenine dinucleotide phosphate (NADPH). The plant uses the energy in these molecules to reduce atmospheric carbon dioxide into three-carbon sugars called triose. These sugars become the molecular building blocks for the organisms while the stored chemical energy in their bonds can be applied to the processes of life.
One of the first major consequences of this energy revolution was a mass extinction. When life first arose on Earth nearly 3.8 billion years ago, the atmosphere was largely carbon dioxide, methane, and water vapor.31 There was no oxygen. Life was adapted for survival in anaerobic conditions. As the first cyanobacteria began producing atmospheric oxygen as a by-product of photosynthesis, it poisoned the microbes whose life strategy relied on anaerobic environments. Called the Great Oxidation Event, the fossil record indicates that this happened 2.4 – 2.1 billion years ago, eventually leading to the rise of organisms who could take advantage of the energy potential of oxygen, a great ancestor of our mitochondria today (they are called “the powerhouse of the cell” for a reason).
A somewhat later consequence of photosynthesis, around 790,000 years before present, there is record of the first human energy revolution, control of fire.32 Archeological evidence from Gesher Benot Ya'aqov in Israel indicates intentional burning of olive and ash wood to cook edible plants, including olives, wild grapes, and wild barley. Building on this development ~10,000 before present, humans began intentionally planting seeds and adopting a more sedentary lifestyle to care for them as they grew. Called the Neolithic Revolution, these events shifted significant populations of humans away from a nomadic hunter-gatherer lifestyle to that of farmers who better were able to increase the amount of energy they had for food and to store surpluses that facilitated trade and social evolution.33 Despite the social and cultural advancements that accompanied this revolution, skeletal records indicate that these populations experienced significant nutritional deficiencies.34 This in part because of reliance on a few domesticated crops like millet, rice, wheat, or maize, which are more vulnerable to climate and disease impacts than the hunter-gatherer lifestyle.35 Food surpluses also introduced the possibility of poisoning from contaminated storage mechanisms, and the social hierarchies that increased class divisions were a source of stress on early farmers.36 Although there have been tremendous improvements in the human capacity to harness photosynthetic energy, we have still only used around five-thousand plant species for food, with less than twenty plant species providing the majority of global calories.37 Today, wheat, rice and corn make up about sixty percent of the calories and fifty-six percent of protein that humans get from plants.38 When considering that the remaining calories from meat were also derived from plants and photosynthesis, brainpower is solar powered.
Mechanical Energy and the Wind
Wind is also a form of solar power. As sunlight enters Earth’s atmosphere, land and water absorb its energy and radiate heat at different rates, indicated by heat capacity, c, in the equation below,
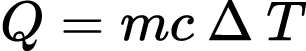
Where Q is the amount of heat energy gained or lost by a material (J), m is the mass (kg), c is the heat capacity (J/gºC), and ΔT (ºC) is the change in temperature.39 Because water has a much higher heat capacity than sand or soil, it takes more energy for the water to heat up, so the air above land heats up more quickly during the day time.40 These higher temperature gases have lower densities, inducing upward air flow that draws in offshore wind. At night, because the land cools more quickly, the cooler, denser air falls, producing air currents that flow out to sea.41
Around 7000 years ago, humans harnessed this energy dynamic to sail the Nile River.42 Another 5,000 years later, approximately 200 B.C.E., engineers in China had developed wind powered water pumps, while reed-woven blades in Persia converted the wind into mechanical energy for grinding grain.43 The equation for converting wind energy to power is formalized in the equation,44
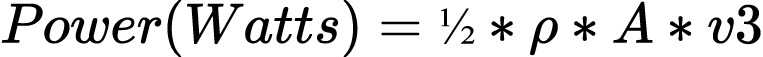
where the Greek letter rho, , is the density of air, whose standard measurement is 1.225 kg/m3; A is the cross-sectional area of the wind in meters squared, m2, essentially the surface area of the whatever is catching the wind. The velocity of the wind, v, is measured in meters per second (m/s).
One world-changing application of this power was for Dutch manufacturing and transportation in the 1600s. Although what we now call the Netherlands was not yet a formal kingdom, the area it now occupies is flat, meaning its rivers were slow and not as amenable to waterwheels which had become fairly widespread at that time in Northern Europe.45 However, because wind is a product of differential heating of land and water, and because almost all of this small country is close to the coast, and because its flatness meant very few obstructions to the wind flow, almost all of the Netherlands had abundant access to wind energy.
When the first windmill was used for sawing timber in 1596, the improvement was dramatic; to hand-saw sixty tree trunks required one-hundred and twenty working days compared with only four or five days using wind-power.46 Other innovations in the Zaan neighborhood north of Amsterdam improved production of canvas, rope, and paper, supporting the Dutch shipbuilding and financial industries, elevating the region to a major industrial center of Europe.47 So valuable was access to this resource that it was protected under feudal law guaranteeing windrecht or “wind rights,” providing for the lease of this energy resource and preventing others from erecting nearby obstructions to it.48
The other source of wind-power innovation was in transportation, a major driver of globalization. Although he died while pursuing this goal, Ferdinand Magellan’s attempt to sail around the world was completed in 1522 by Juan Sebastian del Cano.49 As overseas navigation improved, the Dutch East India company was chartered as a joint-stock company in 1602 to share the risks of sea-trade with Asia.50 Granted an initial twenty-one year monopoly by the unified legislative body of the Netherlands, its charter also gave it the ability to erect forts, raise armies, and make treaties with Asian rulers, a combination of wind and gun powder at the heart of the world’s first military-industrial complex.51 Over its nearly two-hundred years of operation, annual profit averaged about eighteen percent returns, fueling its growth as the largest corporation in history.52 Until its demise in 1796, the company would utilize almost five-thousand ships and send nearly one million Europeans to Asia, more than all other European nations combined.53
The British would inherit this wind-power supremacy after the collapse, and the East India Trading Company came to represent nearly half of global trading in the early 1800s.54 Even the term “trade” in “trade wind” originally meant path or track, but it was their application to foreign commerce that became the predominant association in English.55
Thermal Energy and the Steam Engine
The Industrial Revolution was redefined as an energy transition, one of the most momentous in history, from an organic society fueled by wood, grain, and the muscles of animals to an inorganic economy driven by coal.56
In 1769, James Watt patented his improvements on the Newcomen steam engine.57 This type of engine burned fuel outside a closed system, heating the liquid within and generating pressure that could be converted to useful work. In the U.K., the mechanical energy of these engines was coupled with innovations in cloth manufacturing, increasing productivity and radically changing humanity’s relationship to labor. The resulting Industrial Revolution was so profound that society itself was transformed, moving humanity from a species that was predominantly agrarian to an increasingly urbanized populace removed from “the natural world.”
Where did the energy come from to “fuel” this radical transformation of society? In the U.K., it was predominantly coal. Another form of solar energy, the “buried sunshine”58 of coal is light energy stored by photosynthetic organisms during the Carboniferous period that has been transformed by geological processes over ~360 to 290 million years.59 There are four necessary conditions to change plants into coal. First, the organic material must be fully saturated. During the Carboniferous period, the present day United Kingdom was only 6-8° north of the equator and its humid, tropical climate was subject to frequent marine flooding.60 The water logged organic matter was then decomposed by bacteria that only exist where there is no oxygen.61 The result of this anaerobic process is peat.62 To convert the peat into coal, it must be subject to immense heat and pressure, which happens when layers of sediment bury it deep within the Earth; this removes the water and induces chemical transformations to increase energy density.63 Different chemical structures and qualities of coal can result depending on how much time, heat, and pressure were available during burial.64
When this coal is burned with oxygen, it emits energy, carbon dioxide, and water in a process known as “combustion.”
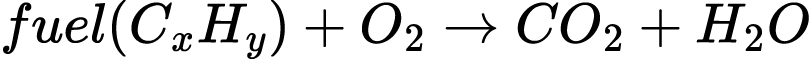
This is the opposite of photosynthesis. Instead of using water and carbon dioxide to capture energy from the sun in the form of sugars, the chemical energy of fuel is released back into the environment in the form of heat and light. Its products are the carbon dioxide and water originally captured by plants before its transformation into fuel. Coal, in particular, also includes sulfur and nitrogen in its molecular structure, resulting in the production of sulfur dioxide (SO2) and nitrogen oxides (NOx) that can pollute air and water.65 Of the four different types of coal, the “cleanest burning” anthracite coals contain 86 – 96% carbon, resulting in greater heat production relative to mass, called energy density, and less air pollution.66
Additionally, combustion is not always a one-hundred percent conversion of fuel into carbon dioxide. This means that partially combusted hydrocarbons (CxHy) can also enter the atmosphere as particulate matter (PM). A form of air pollution that can enter human lungs, its health risks correlate to size measured in microns, i.e. >PM10 are ten microns or greater in diameter and considered “coarse” particles, while PM2.5 is the smallest or “finest” form of particulate matter, can inhaled be into the alveoli of human lungs, and is correlated to increased risk of respiratory and cardiovascular diseases.67

Figure 2. “The Factory at Asnieres” by Vincent Van Gogh, coal fired air pollution in 188768
While records of the first steam engine date to 100 C.E. by Greek inventor, Heron of Alexandria, commercial applications began in 1698 with Englishman Thomas Savery’s steam-powered suction device for removing water from coal mines.69 Following in 1712, Thomas Newcomen increased efficiency by introducing a piston to pull down a beam and draw up water, rather than using the suction of a steam vacuum to move water.70 It was while repairing one of these mining engine that James Watt realized another opportunity to improve efficiency. In the Newcomen model, steam was heated in a cylinder to move a piston, then the cylinder cooled so the water could be heated into steam again for another power cycle.71 This cooling of the cylinder meant it had to be heated up again from a cold start. By introducing a separate condenser, Watt allowed the cylinder to remain hot and the steam to become water again in a separate cold vessel, significantly reducing the amount of coal that needed to be burned per power cycle.
That the English had already begun industrial coal mining was a sign of its long overreliance on wood. By the middle of the 16th century, Britain began to suffer the consequences of its deforestation and by 1700 had switched its primary source of combustion fuel almost entirely to coal.72 This was a partial contribution to why its industrial technology developed so quickly relative to other nations. While steam engines initially were used for draining flooded mines, their application to textile mills and other industrial processes would soon become a major consumer of coal. Early adoption of coal powered steam engines also reduced geographic dependence on water or wind power, which are not available everywhere.73
Not only were steam engines no longer constrained by geography, their impacts would have unprecedented global reach. Combining British colonization with new demands for cotton to fuel textile productivity, “increased exploitation of land in North America, the West Indies, and South America using slave labor imported from Africa.”74 It is estimated that by 1830, the amount of wood, sugar, and cotton imported into Britain would require exploitation of more than ten million hectares of land, nearly twice the amount of agricultural land in the U.K.75 Of those distant exploited hectares, some were forests in Alabama, Mississippi, Louisiana and Texas that were replaced with cotton from 1825 – 1860.76 The human suffering, economic, political, and ecological consequences caused by coal are incalculable.
Electrical Energy and its Induction
Today, steam turbines are frequently used to make electrical energy by use of our next moment, electromagnetic induction. When a conductor moves near a magnet, the magnetic field acts on the electrons inside the conductor, causing them to move. Moving electrons are an electric current. This discovery, first published by Michael Faraday in 1831, was foundational for modern electronics, including inductors, transformers, electric motors and generators.77
Natural philosophers had known since 1820 that there was a connection between magnets and electricity when Danish experimentalist Hans Christian Ørsted published his observation that running a current through a wire would induce a nearby compass to rotate away from true north.78 The next discovery, a few months later, was conducted by the French physicist André-Marie Ampère who showed that two wires could be magnetically pulled together or pushed apart depending on the direction of current flowing through them.79 These two experiments showed that electricity could be used to induce magnetic fields.
What Faraday would show is that the reverse is also true, applying a magnetic field to a wire could produce electricity. In his first example of induction, he carefully wrapped two copper wires around a wooden dowel, connecting one to a battery and the other to a galvanometer for measuring electric current.80 It took several rounds of experimentation with length of wire and battery strength, but he eventually registered a current in the galvanometer when the battery was turned off or on. As previously demonstrated by Ørsted, a wire connected to a battery could create a magnetic field. To produce electricity, the magnetic field must be changing, hence the production of electricity only when the circuit was generating or terminating the magnetic effect.
When he later replaced the wooden dowel with an iron ring, the measured current was significantly higher.81 By creating a more powerful electromagnet with the iron, a proportionally greater amount of electricity was produced. In order to produce a continuous current of electricity, Faraday built on an experiment by the French physicist François Arago. In a demonstration that mystified audiences of 1824, Arago produced a spinning copper disc that could induce a magnetized needle suspended above it to move.82 Faraday set up the copper disc to spin between the two poles of a magnet, connecting its outer and inner edges to a galvanometer. When the disc was spun, a continuous current could be measured.83 The copper conductor was moving through a magnetic field inducing the electrons within to generate a continuous flow of electricity so long as the disc was moving.
This relationship between magnetic flux and amount of electricity produced was later formalized in the Maxwell-Faraday equation,
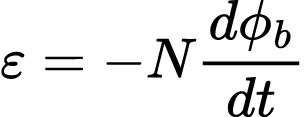
where ε is the electromotive force measured in volts, N is the number of coils in the wire, d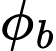 is the change in magnetic flux (Wb), and dt is change over time.84 Because magnetic flux,
is the change in magnetic flux (Wb), and dt is change over time.84 Because magnetic flux, 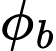 , is a vector having both a magnitude and a direction, change, and thus electromotive force, is induced when it rotates. By attaching an electromagnetic generator to a turbine, anything that spins the turbine can generate electricity. This is the basis of how most electricity is produced via hydropower and wind power. As of 2024, it is also how the majority of the world transforms electricity from chemical energy by using a fossil fueled steam engine to rotate the generator.
, is a vector having both a magnitude and a direction, change, and thus electromotive force, is induced when it rotates. By attaching an electromagnetic generator to a turbine, anything that spins the turbine can generate electricity. This is the basis of how most electricity is produced via hydropower and wind power. As of 2024, it is also how the majority of the world transforms electricity from chemical energy by using a fossil fueled steam engine to rotate the generator.
The first power plant that transformed the kinetic energy of water into electricity was in 1878 at a private home in Northumberland, England and was only used to light a single lamp.85 In the United States, the first hydropower station was in Grand Rapids, Michigan in 1880, with thousands of visitors turning out to see the spectacle of twelve electric street lights.86 The local newspaper, The Grand Rapids Eagle, reported,
Some complained that it was too bright and made their eyes ache and dazzled them; but such had looked directly at the light. When they become more accustomed to the light they will not think of gazing at the lanterns any more than they now do at the sun.
Although initially something a novelty, the capacity for generating industrially productive amounts of energy developed rapidly and became a big motivation for large-scale environmental engineering. In the formula for hydroelectric potential energy,
Hydraulic energy = ρ * g * H * Q
ρ is the density of water (kg/m3), and g is the gravitational acceleration, (m/s2), both of which are relatively constant for freshwater across the globe; what makes the difference in energy generation is water flow, Q (m3/s), and “hydraulic head,” H (m), the height of the water column above the turbine (Figure 3).87

Figure 3. Hydraulic head, H, the height of a static water column above a reference point 88
Although another equation is needed to calculate the amount of potential energy that is converted to shaft power, it indicates that the potential energy of a site increases with higher head, H. This means that many projects around the world require significant inputs of concrete, emitting much carbon dioxide in the process, and have to flood surrounding areas to create deeper rivers and thus higher heads. The negative consequences for the environment surrounding these projects have been significant and vary greatly depending on scale and context.89
Chemical Energy from Coal to Oil
While the negative consequences of hydropower are largely restricted to the area immediately surrounding the riverbed and dam structure, widespread adoption of petroleum has had global consequences. How is petroleum different from coal? For one, coal is solid at room temperature while petroleum is a liquid. These differences are due to chemical structure and what living things were changed in their production. While the organic material that makes coal is largely from woody plants, petroleum mostly results from “slimy” marine plants and algae.90 The reason for their different physical states at room temperature is the extent of their chemical bonding. Coals tend to be more densely connected while petroleum is made of shorter hydrocarbon chains that move more fluidly at lower temperatures.
Another contrast with coal is that it took a little longer to recognize the utility of petroleum products. First published in 1855, Benjamin Silliman Jr.’s, “Report on the Rock Oil, or Petroleum, of Venango Co. Pennsylvania” identified liquid fuels as they were distilled from “rock oil” and some of them burned for illumination.91 While kerosene formed its own highly profitable industry, he found no application for a low boiling fraction, called gasoline, and the useless liquid was dumped into the river.92
Changes to the internal combustion engine would underscore the economic folly of that decision, if not its environmental significance. The first internal combustion engine using liquid petroleum fuel had been patented in 1794. While steam engines were heated from the outside, the newer system combusted fuel with oxygen inside a vacuum to increase pressure of the confined space, directly transforming into mechanical energy by moving a piston rather than losing heat to circulate a secondary liquid. Once these engines became more common through automobile manufacturing in the 1890s, demand for combustion engines and thus their fuel increased also.
Another major turning point in demand for this energy source was the 1911 conversion of the British Navy from coal fired steam ships to oil powered combustion engines under Navy Secretary Winston Churchill.93 Because of its geologic history, coal was abundant in the isles of Great Britain.94 However, there were no reliable sources of domestic oil.95 Chaining the fate of military power to a secure source of imported fuel was one of the most important geopolitical choices of the 20th century. From providing just five percent of global energy in 1915, oil would come to serve forty percent of the world’s total energy needs in just sixty years.96
What Will the Next Moment Look Like?
The history of energy shows us that the old has often been overshadowed by the new, but rarely has a legacy form truly vanished. History has a way of sticking around.97
Coal, oil, natural gas and other fossil fuels are so named because they are the result of geological processes acting on organic matter. Burning these hydrocarbons with oxygen produces carbon dioxide (CO2), a greenhouse gas that absorbs infrared radiation. Nearly 70% of all human produced carbon emitted since 1870 has been from combustion of fossil fuels.98 While life on Earth has long benefited from a stable concentration of CO2 to warm the atmosphere, this rapid increase in CO2 concentration since the Industrial Revolution has increased average global temperature.99 For as large as the Earth and its oceans are, to increase its temperature by even 1 °C is an enormous change in the amount of energy stored around our planet. The consequences of this extra energy have been termed “climate change.” Weather patterns are changing, ice sheets are melting, storms or droughts are becoming more extreme, habitable zones for animals are shifting, and the whole system is less predictable, itself a serious risk in a globalized economy.100
One effort to reduce the impacts of these changes is finding replacements for fossil fuels. Electrification and battery storage are promising technologies, but “uses of energy that appear rational or even inevitable in boardrooms or legislatures are often violent, bloody, and irrational on the ground.”101 The most efficient battery technologies today rely on critical minerals such as lithium and cobalt, which have their own geopolitical consequences, including child labor violations,102 trade monopolies, as well as conflict in Afghanistan and Bolivia.103 As we attempt to resolve one technological crisis with another technological solution, it is important to remember that these transitions have been messy, and tend toward adding new forms of energy to the existing mix rather than replacing the old ones as transition might imply.104

Comments: