Strategies & Activities
Lesson 1: Background and Approach
As we develop the scaffold of knowledge for our students, there are key concepts that are vital to their success in Chemistry. Being able to identify reactants, products, and the reaction type are critical; understanding how to calculate the Gram Formula Mass (GFM) for a single atom or complex molecule is also a necessity. In conceptual order, the recommended scaffolding approach is Reaction Concepts → Reaction Types → Atom Count in molecules → Law of Conservation of Mass → GFM → Atom Economy.
Reaction Types can generally be broken into six types (make sure to let your students know two more types of reactions, dissociation and nuclear, will be covered later in the year). Please note that in the examples given for Table A below, A, B, C and D represent a 'placeholder' for Elements from the Periodic Table of Elements in the first four reaction types, while H represents Hydrogen, O Oxygen, C Carbon, A Acid and B Base in the Combustion and Acid-Base reactions:
Table A - Reaction Types
| Reaction Types | Algebraic Representation |
| Synthesis | A + B -> C |
| Decomposition | AB -> A + B |
| Single-Replacement | A + BC -> AC + B |
| Double Replacement | AB + CD -> AD + CB |
| Combustion | (C + H) + O2 -> CO2 + H2O |
| Acid-Base | HA + BOH -> BA (salt) + H2O |
Recognizing the number of atoms within a molecule is also an essential understanding for Chemistry. Numbers in front of the molecule (i.e., 3 in 3NaCl) are called coefficients; subscripts follow the particular atom or molecule that they coincide with (i.e., 2 in H 2O). If a molecule is made up of one element, than variations of texts may refer to it as an atom or a molecule. Table B gives an example of six numerical variations that commonly occur when counting atoms:
Table B - Six Numerical Variations for Counting Atoms
| C Means there is 1 Carbon Atom (no number next to atom means that there is 1 atom) | 3C Means that there are 3 Carbon Atoms (3*1) | 4H2 Means that there are 8 Hydrogen Atoms (4*2) |
| NO3 Means that there are 3 Oxygen Atoms and 1 Nitrogen Atom | 2NO3 Means that there are 6 Oxygen Atoms (2*3) and 2 N Atoms (2*1) | 4(NO3)2 Means that there are 24 Oxygen Atoms (4*3*2) and 8 Nitrogen Atoms (4*1*2) |
The Gram Formula Mass (GFM), also commonly referred to as molecular mass, molecular weight, or formula weight, is determined by adding the atomic mass units (amu) found on the periodic table of all the atoms in a molecular compound. This GFM is the amount of grams of substance in one mole; thus, the units will be in grams per mole, or annotated as g/mol. When calculating the GFM, you add the total mass for all the atoms within the molecule. Using the six variations from Table B, Table C below shows the calculation of Gram Formula Mass for each molecule:
Table C - Gram Formula Mass Variations
| C = 12 g/mol C – 12 amu x 1 atom |
3C = 36 g/mol C – 12 amu x 3 atoms |
4H2 = 8 g/mol H – 1 amu x 4x2 atoms |
| NO3 = 62 g/mol N –14 amu x 1 atom N = 14 g/mol O3 –16 amu x 3 atoms O = 48 g/mol |
2NO3 = 124 g/mol N –14 amu x 2x1 atoms N = 28 g/mol O3 –16 amu x 2x3 atoms O = 96 g/mol |
4(NO3)2 = 496 g/mol N –14 amu x 4x1x2 atoms N = 112 g/mol O3 –16 amu x 4x3x2 atoms O = 384 g/mol |
Once you understand reactions, reaction types, how to count atoms within molecules, and how to calculate the GFM, you have the scaffold knowledge to understand and calculate the Percent Atom Economy. The Percent Atom Economy is the percent "of what you put into your pot [that] ends up in your product." 3 The formula for calculating the Percent Atom Economy is:

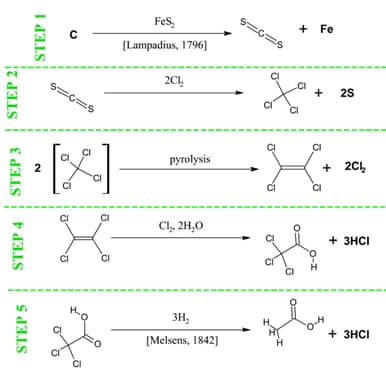
This lesson will teach 4 objectives and review 4 topics. Multiple Step Reactions, Gram Formula Mass, Atom Economy, and E-factors will be taught. Reaction types, some basic laboratory safety techniques, Law of Conservation of Mass, and vocabulary will be reviewed. Before handing out the 'Analysis of Acetic Acid Production Sheet', pour 5 mL of vinegar into each of 5 test tubes (make sure not to let the students see you pour it). Review with the students how to properly sniff from an unknown substance (wave hand over the test tube opening and waft towards your nose), as well as how to hold a test tube (using test tube tongs). Ask them what they think is in the test tube. Use words that are easily identifiable with acids so that you can scaffold their understanding of acids and bases as the year progresses. Have them write down quantitative and qualitative properties of the liquid. Have the students classify those properties as physical or chemical. If needed, use a pH paper to test the pH (which should be well below 7.0 since it is an acid). Mention that acetic acid is known in the Chemistry world as ethanoic acid, and is with the carboxylic acid family; it is used to wash medicines such as ibuprofen, used in soft drink bottles, and is commonly found in wood glue. Acetic acid can be made synthetically from petrochemical feedstocks, or from biological sources. After some discussion, let the students know that the substance in the test tube is vinegar, and that it is a biologically sourced form of diluted acetic acid. 4 With this information covered, have the students look at Table E and while you describe the multiple steps that it takes to make Acetic Acid, emphasize that the stick and ball diagrams represent the same thing as the stick diagrams, and that both represent the molecular abbreviations. Hand out a modified version of Table D (Table D has the answers filled in for your convenience, but remove as necessary for your students) and have the students complete the sheets individually or in pre-selected groups of two.
Table D - An Analysis of Acetic Acid Production
| An Analysis of Acetic Acid Production – Instructions (Multiple Step Reaction and Reaction Types) Fill out the table below with the step#, reaction type, reactants, catalysts (if any), products, what chemicals we are keeping, and which we are wasting. Then, calculate the Gram Formula Mass (GFM) for each molecule (don’t forget your units!). Proceed to calculate the total GFM of your reactants, products, and wasted molecules for each step. Finally, complete the atom economy calculation. | |||||||||||||||||||||
| Step # | Reaction Type | Reactant Molecules | Catalyst | Product Molecules | Atoms Kept | Atoms Wasted | |||||||||||||||
| 1 | Single Repl. | C, FeS2 | CS2, Fe | CS2 | Fe | ||||||||||||||||
| 2 | Single Repl. | CS2, Cl2 | CCl4, S | CCl4 | (2)S | ||||||||||||||||
| 3 | Decomp. | CCl4 | Heat | C2Cl4, Cl2 | C2Cl4 | (2)Cl2 | |||||||||||||||
| 4 | Single Repl. & Dbl Repl. | C2Cl4, Cl2, H2O | C2Cl3OOH, HCl | C2Cl3OOH | (3)HCl | ||||||||||||||||
| 5 | Single Repl. | C2Cl3OOH, H2 | CH3COOH, HCl | CH3COOH | (3)HCl | ||||||||||||||||
| Calculate the Gram Formula Mass | |||||||||||||||||||||
| C: 12 g/mol | Fe: 56 g/mol | S: 32 g/mol | H2: 2 g/mol | CS2: 76 g/mol | Cl2: 70 g/mol | CCl4: 152 g/mol | |||||||||||||||
| FeS2: 120 g/mol | C2Cl4: 164 g/mol | H2O: 18 g/mol | HCl: 36 g/mol | C2Cl3OOH: 162 g/mol | CH3COOH: 60 g/mol | ||||||||||||||||
| Calculate Total Gram Formula Mass | |||||||||||||||||||||
| Step 1: | Reactants: 132 g/mol | Products: 132 g/mol | Products Wasted: Fe | ||||||||||||||||||
| Step 2: | Reactants: 216 g/mol | Products: 216 g/mol | Products Wasted: 2S | ||||||||||||||||||
| Step 3: | Reactants: 304 g/mol | Products: 304 g/mol | Products Wasted: 2Cl2 | ||||||||||||||||||
| Step 4: | Reactants: 270 g/mol | Products: 270 g/mol | Products Wasted: 3HCl | ||||||||||||||||||
| Step 5: | Reactants: 168 g/mol | Products: 168 g/mol | Products Wasted: 3HCl | ||||||||||||||||||
| What pattern(s) do you notice? The reactants weigh the same as the products (Law of Conservation of Mass). | |||||||||||||||||||||
| Calculate Atom Economy | |||||||||||||||||||||
| Final Product Formula: CH3COOH | Final Product GFM: 60 g/mol | ||||||||||||||||||||
| Total GFM of all products: 1090 g/mol | Atoms Wasted: Fe, (2)S, (2)Cl2, (6)HCl | ||||||||||||||||||||
| Atom Economy Formula Inputs: (Final Product GFM÷(Total GFM of final product + wasted products))x100 = (60÷(60+476))x100 = (60÷536)x100 = 11.2 % | |||||||||||||||||||||
| Atom Economy (%): 11.2 % | |||||||||||||||||||||
Table E - Acetic Acid Production - Stick Diagram
An Analysis of Acetic Acid Production (Multiple Step Reaction, Reaction Types, E-factor and Atom Economy)
Lesson 2: Background and Approach
Basic Stoichiometric Dimensional Analysis is an essential survival skill in Chemistry. It allows us to use physical and chemical properties of chemistry to mathematically manipulate numbers and units to solve simple and complex problems. Although stoichiometry is one of the fundamental skills in Chemistry, it is an essential math skill as well that, if mastered, can carry on with our students through their lifetime; unfortunately, getting our students to make that transference can sometimes be difficult.
To understand stoichiometry, there are 5 essential understandings that you need to have. First, numbers and units do not necessarily follow each other, yet both are always needed to properly express something; 4 quarters is 1 dollar, but 4 dollars is not 1 quarter, and 4 or dollars by itself means nothing. Second, any number over 1 is still that number. Third, a quantity divided by itself is equal to one, including units and numbers; 4 quarters divided by 1 dollar is equal to 1. Fourth, any quantity multiplied by 1 is itself. Finally, it is important to conceptualize that units units = 1, and that units units = units.2 Knowing how to combine and dissociate numbers and their respective units to find answers in secondary numbers and units will allow you to complete this lesson, and develop a good fundamental understanding for the process and understanding of stoichiometry that will be used through Chemistry and beyond.
Table F below has 5 examples of stoichiometric conversions, as well as numerical descriptions of the description from the paragraph above.
Table F - Stoichiometric Conversion Examples

While the goal of this lesson is to teach the fundamentals of stoichiometry, the heart of this lesson is in the shock factor impact that I want the students to have. Real world examples of the amounts of pollutants that we have created will be used and sourced. Rather than telling the students that we are on a collision course, I will allow them to come to the conclusion that there is a problem (i.e., Based on these variables, how many gallons of plastic bottles are thrown in our dumps in 2 weeks? 5 months? 1 year?) To allow the students to visualize how the impact will affect them, the space requirements of the waste generated will also be calculated. (i.e., Based on these variables, how much space in landfills will be needed in the next 10 years? How many times could you cover the surface area of the earth with this waste? 5 Conversions will be utilized throughout these questions so that students can grasp the concepts of converting, and overcome the 'memorization'/'calculator only' process of converting.
Once students begin grasping the severity to our planet that the facts represent, we will focus on learning how to express this information. What does this mean? What can be done to solve this problem? Is money going to be the limiting factor in the years to come, or is water, air, oil, gas, fuel, or electricity? 6 This stoichiometry lesson will end with reviewing and having the students quantify the energy requirements of our planet and various existing solutions. The students will begin understanding that there is a whole world of opportunity in the future for people involved in the harnessing of Energy; harnessing the world's energy will bring opportunities to Change The World. After the lesson, we will review the 12 principles of Green Chemistry.
As an interim between Lessons 2 and 3, not covered in this unit, students should be exposed to the concepts of balancing equations numerically and using visual diagrams. Students should be proficient in mathematically balancing more complex reactions. The Law of Conservation of Mass should be reviewed and taught as a method to tell if 'something is missing' when the students are calculating their own products. A website which is an excellent resource for these concepts is http://www.explorelearning.com and is highly recommended.
Lesson 3: Background and approach
At this point, the foundation for atom economy has been covered, although it is conceivable that the students will not yet have a good conceptual grasp of the concept. Lesson 3 will reinforce the concept of Atom Economy and introduce the concept of E-Factor using Pfizer's 'Economizing with the Atom' exercise (appendix B). The Environmental Factor (E-Factor) is a generic metric that can be used to measure the environmental effectiveness of the production of a substance. Roger Sheldon is recognized as the one who introduced this formula which is:

The 'Economizing with the Atom' exercise will help students visualize atom economy and the E-Factor. As an extension to the lesson, have students generate a list of "Life's Products We Use". Have the students break down the list into the 4 categories from the exercise.
| Category | E-Factor | Life’s Products We Use |
| Petrochemicals | 0.1 | Nail Polish |
| Bulk Chemicals | 1-10 | Plastic Water Bottles |
| Fine Chemicals | 100 | iPOD Touch Screen |
| Pharmaceuticals | 250 | Antibiotics |
Then have the students consider the life cycle of those products. Have the students answer the following question - Does the E-Factor of 'Life's Products We Use' change over the life cycle of the products? Explain your answer (Sample Answer: Yes, the E-factor changes over the life cycle of a product. After manufacturing, the product needs to be shipped, which adds to the waste. The product is transported with packaging that may also get thrown away after use.). 7 This exercise will be used to express and help students conceptualize how much waste is created by the various products they use on a daily basis, AFTER production of the item. Discussions will occur as to the impact that the consumer driven society we live in is having on our world, its resources, and our existence.
Lesson 4: Background and Approach
A Case Study will be done on Ibuprofen, and the innovative way in which it is now being produced. 8 The pedagogy of the case study will be unique in that it integrates an interactive 'whodunnit' lesson to teach the students how to analyze the wasted chemicals that the original process created, while looking at other properties. Then, a straightforward analysis will be conducted as to the new methodology and its impact on the world.
Ibuprofen was first patented and introduced in 1969 by the Boot Pure Drug Company, who developed a six step process of developing this 'wonder drug' originally named Brufen. They started with a petroleum based compound known as 2-methylpropylbenzene, and through this six step process created Brufen (also known as 2-(p-Isobutylphenyl)propionic acid). 9 Table H below shows this original process, which became known as the Boot Process. Unfortunately, the Atom Economy was less than 40%, which meant that for every 40 grams made, 60 grams was ending up as toxic waste. In 1997, the BHC Company (now BASF Global) received the 1997 EPA's Presidential Green Chemistry Challenge Award for a new method of synthesizing Ibuprofen. The new method, called the Hoechst Process (see Table I), makes ibuprofen in three steps, and achieves a Percent Atom Economy of greater than 77%, meaning that for every 77 grams of ibuprofen that is produced, now only 23 grams is made up of waste (and the acetic acid wasted from step one can actually be recycled to make more ibuprofen, bringing the actual Percent Atom Economy closer to 90%). 10
The lesson is jumpstarted with the activity 'A Murder Most Foul'. Student instructions for this activity are described in Table G1; the known facts about 'The Headache' are all accurate statements about Ibuprofen. The only paper evidence found on 'The Headache' is his 'formula book' which is Table H. Table G2 has 14 "wasted chemicals" that have clues as to what step in the Boot Process they came from. Table G2 has the answers in the conclusion section that the students will fill out. The Forensic Scientist Data Sheets are accurate, and provide plenty of opportunities to review concepts such as chemical/physical properties, Diatomic Molecules, GFM, and naming conventions. 11 I suggest finding some manipulative elements and creating 'stations' in your room outlined in tape to emphasize what happened to the 14 elements. The purpose of this exercise is two fold. First, this exercise should help the students conceptualize the ibuprofen formation process (via the older Boot Process). While reviewing elemental properties, the students should then be able to isolate the wasted elements and correlate them to the correct step that they were 'wasted' in, and be able to calculate the final atom economy. When this activity is complete, go over the Boot Process in detail, stopping to describe which elements are wasted in each step (see Table H). Have the students verify their answers. Discuss the following: Since 30 million pounds of ibuprofen are produced each year, how much waste is produced by this method? (Answer: % Economy Calculates to 40.2 %, so the amount wasted using this process equates to 59.8%. [30,000,000 lbs./yr. 0.598 = 17,940,000 lbs. of waste generated per year]) Discuss the implications of this, and allow students time to absorb the enormity of the problem.
Next, explain the 'Green' method of making Ibuprofen, showing the students the Hoechst Process (Table I). Calculate the atom efficiency of this process. Using the same 30 million pounds per year example, calculate the amount of waste produced each year using the new process; the figure is huge, but significantly smaller than the Boot Method. (Answer: % Economy Calculates to 77.4 %, so the amount wasted using this process equates to 22.6%. [30,000,000 lbs./yr. 0.226 = 6,780,000 lbs. of waste generated per year]) Also, make sure to mention that using this new process, the acetic acid generated from Step 1 of the reaction can actually be recycled over and over again to create more ibuprofen. 12
Table G1 - A Murder Most Foul Activity
A Murder Most Foul
The District Attorney's Office Needs Your Help to convict the Head of the Brufen Family, Ibu Profin Brufen, a.k.a., "The Headache". The Headache is an organized, synthesized chemical drug boss that MURDERED many chemicals in his rise to the top. We have the broken families of chemicals (or what's left of their bodies, in most cases). We have some qualitative and quantitative evidence that the CSI forensic scientists have gathered. Your mission is to: 1.) Decipher the only paper evidence found on "The Headache" (from Table H), 2.) Conduct autopsy forensic research to prove quantitatively and without a doubt what chemicals were killed and in what step, 3.) Present this information to Judge Teacher to see if there is enough quantitative evidence to get a conviction, and 4.) Calculate "The Headache's" murder ratio, known as atom economy, so that we can accurately describe how murderous he was. Use the evidence given below to guide you:

Known Facts
- Born 1969, England
- Resides in Bishop, Texas (since 1992)
- Known for: Drug Production 30 million pounds per year
- Street Names (a.k.a.'s) Advil, Motrin, Nuprin, Proflex, RD13621
- Weight: Needs to be figured out
- What makes him melt: 77-78 ?C
- Stable Drug KingPin
Table G2 - A Murder Most Foul: Forensic Scientist Data Sheets
Forensic Scientist Data Sheets
| Item No.: | 1 | Date Found: | 8/15 | Partial/Full Body: | Partial | ||||||
| Location Found: | High Gases Lane | GFM (g/mol): | 1 | ||||||||
| Other: | Est. Original GFM (g/mol): | ||||||||||
| Chemical Properties: | Flammable | ||||||||||
| Physical Properties: | Gas @ 77 ËšF, No Color | ||||||||||
| Qualitative Observations: We suspect this atom was cut off from ‘The Headache’ himself in an attempt to give him a bonding connection on his rise to power. | |||||||||||
| Conclusion: Molecule is H from Step 1. GFM = 1 g/mol. Hydrogen is high on the Periodic Table, and is a Gas @ 77 ËšF, has no color, and is extremely flammable. The ibuprofen process starts off with a C10H14 molecule that releases this Hydrogren Atom to begin the synthesis. Also, the date is indicative of the first step of the process. | |||||||||||
| Item No.: | 2 | Date Found: | 8/15 | Partial/Full Body: | Partial | ||||||
| Location Found: | Acetic Anhydride Circle | GFM (g/mol): | 59 | ||||||||
| Other: | Est. Original GFM (g/mol): | ||||||||||
| Chemical/Physical Properties: | |||||||||||
| Qualitative Observations: 1 Double Bond amongst the mutilated atoms | |||||||||||
| Conclusion: Molecule is C2H3O2 from Step 1.GFM = 59 g/mol. The ‘mutilated double bond’ came from the C4H6O3, and this molecule is called Acetic Anhydride. Also, the date is indicative of the first step of the process. | |||||||||||
| Item No.: | 3 | Date Found: | 8/16 | Partial/Full Body: | Full | |||||
| Location Found: | Luv Carbonyl Lane | GFM (g/mol): | 68 | |||||||
| Other: | Est. Original GFM (g/mol): | 68 | ||||||||
| Chemical Properties: Likes to take H’s from alpha Carbons & Inflammable | ||||||||||
| Physical Properties: Inorganic Salt | ||||||||||
| Qualitative Observations: The Drug Boss didn’t even try to hide this chemical body… he just left it used and wasted into 9 atoms lying on the streets. | ||||||||||
| Conclusion: Molecule is NaOC2H5 from Step 2. GFM = 68 g/mol. It is an inorganic salt called a Carbonyl. The 9 atoms are 1-Na, 1-O, 2-C, and 5-H. It is the only molecule that gets used 100% in the Boots Process. The date (one day later than the previous items) indicates the second step of the process. | ||||||||||
| Item No.: | 4 | Date Found: | 8/16 | Partial/Full Body: | Partial | ||||||
| Location Found: | Light Gas Vista | GFM (g/mol): | 1 | ||||||||
| Other: | Est. Original GFM (g/mol): | ||||||||||
| Chemical Properties: Flammable | Physical Properties: Density: 0.082 g/liter | ||||||||||
| Qualitative Observations: Found trying to connect its one electron to an atom with 7 valence electrons in its outer shell | |||||||||||
| Conclusion: Molecule is H from Step 2. GFM = 1 g/mol. Hydrogen is a light flammable Gas that has a density of 0.082 g/liter. The H is ‘cut off’ along with the Cl (which has 7 valence electrons in its outer shell). The date indicates Step 2. | |||||||||||
| Item No.: | 5 | Date Found: | 8/16 | Partial/Full Body: | Partial | ||||||
| Location Found: | Halogen Circle | GFM (g/mol): | 35 | ||||||||
| Other: | Halogen | Est. Original GFM (g/mol): | |||||||||
| Chemical Properties: | Melting Point: -150.7 F | ||||||||||
| Physical Properties: | Green | ||||||||||
| Qualitative Observations: | Watch out with this one, when scientist #3 sniffed it slightly, he got very ill. | ||||||||||
| Conclusion: Molecule is Cl from Step 2. GFM = 35 g/mol. Chlorine is a green Halogen Gas that has a melting point of -150.7 ËšF. Chlorine gas is poisonous and even sniffing small quantities can make you ill. The date indicates Step 2. | |||||||||||
| Item No.: | 6 | Date Found: | 8/17 | Partial/Full Body: | Partial | |||||
| Location Found: | Hydronium Lane | GFM (g/mol): | 1 | |||||||
| Other: | Was attracting my negativity | Est. Original GFM (g/mol): | ||||||||
| Chemical/Physical Properties: | ||||||||||
| Qualitative Observations: Diatomic Tendencies | ||||||||||
| Conclusion: The H ion from Step 3. GFM = 1 g/mol. Hydrogen ion is usually associated with H3O+, a hydronium ion. H has diatomic tendencies, and because it has a positive charge, it tends to attract negative particles. The date indicates Step 3. | ||||||||||
| Item No.: | 7 | Date Found: | 8/17 | Partial/Full Body: | Partial | ||||
| Location Found: | Evian Lane | GFM (g/mol): | 16 | ||||||
| Chemical Properties: Density = 1.308 g/liter | Est. Original GFM (g/mol): | ||||||||
| Physical Properties: Gas at room temperature | |||||||||
| Qualitative Observations: Diatomic Tendencies | |||||||||
| Conclusion: The Atom is O from Step 3. GFM = 16 g/mol. Evian is a type of water, hinting that the O came off the water molecule. Oxygen’s density is 1.308 g/liter, is a diatomic molecule, and is a gas at room temperature. The date indicates Step 3. | |||||||||
| Item No.: | 8 | Date Found: | 8/17 | Partial/Full Body: | Partial | ||||||
| Location Found: | Ethyl Formate Lane | GFM (g/mol): | 73 | ||||||||
| Other: | 10 atoms had one bonding point | Est. Original GFM (g/mol): | |||||||||
| Chemical/Physical Properties: | |||||||||||
| Qualitative Observations: Had a piece of paper on 1 atom which read “I’m on top of the world, ma!” | |||||||||||
| Conclusion: The Molecule is COOC2H5 from Step 3. GFM = 73 g/mol. This molecule is known as an Ethyl Formate, and is often written as COOEt, where Et represents C2H5. The 10 atoms that form this molecule with single bonds are 3-C, 2-O, and 5-H. The ‘on top of the world’ comment is due to the COOEt which looks like it is on top of a pyramid (albeit sideways). The date indicates Step 3. | |||||||||||
| Item No.: | 9 | Date Found: | 8/18 | Partial/Full Body: | Partial | |||||
| Location Found: | Hydroxylamine Drive | GFM (g/mol): | 2 | |||||||
| Other: | 2 atoms - identical | Est. Original GFM (g/mol): | 33 | |||||||
| Chemical/Physical Properties: | ||||||||||
| Qualitative Observations: White, crystalline residue near site where found | ||||||||||
| Conclusion: The atoms are 2 H’s (note: not H2) from Step 4. GFM = 2 g/mol. These 2 identical Hydrogen atoms come from a molecule known as a Hydroxylamine, which is a white crystalline residue. The date indicates Step 4. | ||||||||||
| Item No.: | 10 | Date Found: | 8/18 | Partial/Full Body: | Partial | |||||
| Location Found: | Ibu Swamp Lane | GFM (g/mol): | 16 | |||||||
| Other: | Est. Original GFM (g/mol): | |||||||||
| Chemical/Physical Properties: | ||||||||||
| Qualitative Observations: Signs of a previous double bond | ||||||||||
| Conclusion: The atom is O from Step 4. GFM = 16 g/mol. This atom came off of C13H18O, where it had a double bond. The Ibu Swamp Lane is a clue, since the O comes off of the big molecule that is forming Ibuprofen. The date indicates Step 4. | ||||||||||
| Item No.: | 11 | Date Found: | 8/19 | Partial/Full Body: | Partial | |||||
| Location Found: | 1 block from Double Bond Lane | GFM (g/mol): | 1 | |||||||
| Other: | Est. Original GFM (g/mol): | |||||||||
| Chemical/Physical Properties & Qualitative Observations: | ||||||||||
| Conclusion: The atom is H from Step 5. GFM = 1 g/mol. This atom came off of the C==N double bond, and item no. 12 is also ‘1 block from Double Bond Lane’; this is the clue to recognize that this H came from Step 5. The date also indicates Step 5. | ||||||||||
| Item No.: | 12 | Date Found: | 8/19 | Partial/Full Body: | Partial | |||
| Location Found: | 1 block from Double Bond Lane | GFM (g/mol): | 17 | |||||
| Chemical Properties: Has a negative charge lingering | ||||||||
| Physical Properties: | ||||||||
| Qualitative Observations: Had a tattoo ~Attach yourself to those that are a gas of laughs and you will live on the edge~ | ||||||||
| Conclusion: The molecule is OH from Step 5. GFM = 17 g/mol. This molecule came off of the C==N double bond, and item no. 11 is also ‘1 block from Double Bond Lane’ as a clue. OH as an ion is called Hydroxide and has a negative charge. The ‘attach yourself to’ tattoo is referring to the Nitrogen the OH was attached to; Nitrogen is also known as laughing gas. The date indicates Step 5. | ||||||||
| Item No.: | 13 | Date Found: | 8/20 | Partial/Full Body: | Partial | |||||
| Location Found: | Triple Bond Lane | GFM (g/mol): | 14 | |||||||
| Other: | Est. Original GFM (g/mol): | |||||||||
| Physical Properties: Nonmetallic | ||||||||||
| Qualitative Observations: | ||||||||||
| Conclusion: The atom is Nitrogen (N) from Step 6. GFM = 14 g/mol. This nonmetallic atom came off of the only triple bond in the process. The date indicates Step 6. | ||||||||||
| Item No.: | 14 | Date Found: | 8/20 | Partial/Full Body: | Partial | |||||
| Location Found: | Rain Lane | GFM (g/mol): | 3 | |||||||
| Other: | 3 separate atoms | Est. Original GFM (g/mol): | ||||||||
| Chemical/Physical Properties: | ||||||||||
| Qualitative Observations: 3 similar atoms appear to have been passing by connected to their original molecule (estimated at 18 g/mol) when ‘The Headache’ wasted them. | ||||||||||
| Conclusion: The atoms are 3 Hydrogens (H) in Step 6. Total GFM = 3 g/mol. They all came off of water (H2O) molecules whose GFM is 18 g/mol. The Rain Lane is a clue to lead the students to think ‘water molecules’. The date also indicates Step 6. | ||||||||||
Table H - Original Boot Process
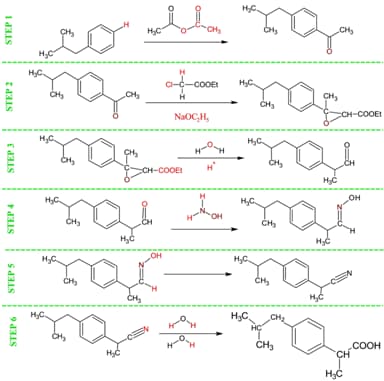
Table I - New "Greener" Ibuprofen Process
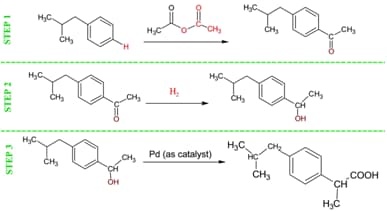

Comments: