Appendix
A.The 12 Principles of Green Chemistry
- It is better to prevent waste than to treat or clean up waste after it is formed.
- Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.
- Wherever practicable, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
- Chemical products should be designed to preserve the efficacy of function while reducing toxicity.
- The use of auxiliary substances (e.g. solvents, separation agents, etc.) should be made unnecessary wherever possible and innocuous when used.
- Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure.
- A raw material or feedstock should be renewable rather than depleting wherever technically and economically practicable.
- Unnecessary derivatization (blocking group, protection/de-protection, and temporary modification of physical/chemical processes) should be avoided whenever possible.
- Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
- Chemical products should be designed so that at the end of their function they do not persist in the environment and break down into innocuous degradation products.
- Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
- Substances and the form of a substance used in a chemical process should be chosen so as to minimize the potential for chemical accidents, including releases, explosions, and fires.
B. Pfizer's Economizing with the Atom Activity
Economizing with the Atom
Based upon the E-factor Lesson developed by Irv Levy
Educational Goal: To provide an understanding of atom economy and how it is used in chemical processes and how it can be applied to everyday life.
Student Objectives: Students will…
- Understand principle 2 of green chemistry
- Perform an exercise which has them practice atom economy
- Relate the exercise to chemical processes
Materials (for a class of 32):
- One large bag of M&Ms
- Digital scale
- Package of individually wrapped potato chip portions
- Optional: plastic gloves
Time Required: 45-60 minute class period
Standards Met: S1, S2, S5, S6, S7
Green Chemistry Principles Addressed: 2
Prep: Prepare five baggies of M&Ms as shown below.
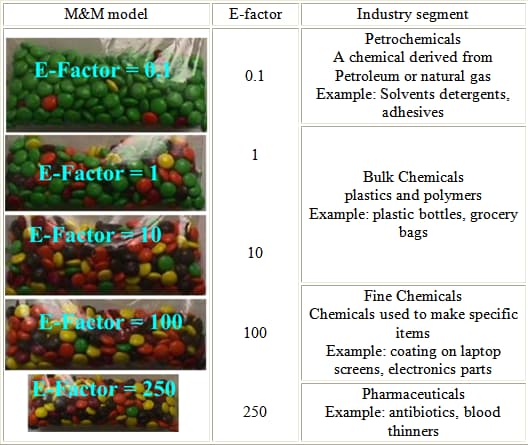
Procedure:
- Project slide # 1
- E-factor ≡ mass of waste ÷ mass of product
- Ask the students for 4 volunteers who are ready for a snack.
- Ask the volunteers to come to the front of the class.
- Tell students that the goal of this experiment is to satisfy your need for a quick snack and to test out our equation using these individually packaged potato chips.
- Ask the students to unpack the potato chips and make a pile of chips and a pile of waste. You may want to have students use gloves at this stage so that they can eat the chips after.
- Ask the students to find the mass of the chips and then the mass of the packaging and record the totals on the board.
- Explain to the students that we are not finished yet as we haven't used a key component in green chemistry principle # 4 and tested to see if this product retains it's efficacy. Therefore the volunteers will need to eat the chips and tell us if they satisfy their craving for a snack.
- While they eat, have another student come up and solve the E-factor equation for the potato chips.
- Debrief with students as to how well the product performed in regards to it's e-factor. Make sure you include a discussion of why there was all that packaging and how that is what the person designing the product decided you needed in order to deliver the product you wanted.
- Ask students how the e-factor of this product might be improved.
- Ask students to get into groups of two or three.
- Tell the students that actually this formula is used by green chemists to evaluate chemical processes so at this point we would like to give you all a bag of chemicals.
- Hand out a small bag of M& Ms to each group of students.
- Explain that this bag of M&Ms is very special but that unfortunately the only ones that you can use are the green ones.
- Ask students to separate the green M&Ms from the other colors and make two piles. It turns out that green M& Ms have been discovered to be a key component in a revolutionary technology that helps to make hologram images come out of cell phones so that people can see each other when they chat.
- The green part is actually the chemical that you need.
- Ask students to calculate the e-factor for the bag of M&Ms
- Ask the students to tell you whether they are OK with having all this waste in order for them to have hologram images of their friends come out of their cell phones.
- Tell the students that you are going to give them another equation to help them be creative.
- Put the following equation on the board.
- E-factor = (mass of inputs - mass of outputs) ÷ mass of product
- Ask students to creatively come up with ways that they could reduce the e-factor.
- Discuss with students their possibilities, real or imagined.
- Show students the pre-prepared bags of M&Ms and explain how these bags represent chemical processes.
- Now tell the students that you know, they know that isn't real but that these techniques are really being used in industry.
- Ask students if any of them use Ibuprofen. You may have to prompt them with over-the-counter brand names of the drug.
- Project the slide of the Ibuprofen diagram number one on the accompanying PPT presentation.
- Talk through the process with the students.
- Explain that at the time Ibuprofen was invented it was a big breakthrough for people suffering from joint and muscle pain, it was originally invented to help arthritis patients. Sounds great right? Until you look at how much of the process created waste.
- Go to slide two where the graphic is highlighted with color
- Then highlight for the students that the green parts are the processes that are incorporated in the product and the brown is the waste.
- Put one ibuprofen pill on the desk (preferably green in color) and one brown ball that is roughly half the size again, and explain that this is the ratio.
- Ibuprofen was manufactured this way and sold over the counter between roughly 1980 and 1990 with around 46 tonnes of ibuprofen sold in the UK and Northern Ireland per year during that time.
- With the old method, that means that there would be 68.72 tonnes of waste = 69 female walruses of waste.
- Ask the students to tell you how many tonnes of waste that would be over 10 years.
- The process was changed in 1990 through the use of green chemistry techniques and specifically Atom Economy.
- Show the next slide with the new process. Ask the students to tell you what they immediately see as being different.
- Talk the students through the new process. Point out that the acetic acid used in step one of the new process is recovered and used over again.
- Point out that the current and better method = 13.33 tonnes of waste, 13 female walruses. (show slide 5)
- The new method: If you count the recycling of the single main waste product, a mere 1% of the building block atoms result as waste. (show slide 6) The process also replaced a six-step by a three step process, aiding energy efficiency (principle 6) and simplifying real-time analysis for pollution prevention (principle 11).The waste before was mostly landfilled.
- Ask the students to refer back to the 12 principles and even though this is a green chemistry success story ask them to tell you why this process doesn't totally adhere to the 12 principles of green chemistry. Answer: Recycling is not really a green chemistry principle. It is better not to create waste in the first place.
- Explain that in the 60s when Ibuprofen was first synthesized, chemists weren't thinking about toxicity and lifecycle, so they just made a wonder drug and called it good. These days chemists are having to go back and fix a lot of these things. True green chemists, like us, look at the processes and materials through a green eye from the very beginning.
- Scientists are also looking at Bi-products as well. Chemicals that have been developed in the past have bi-products and scientists are looking at innovative ways to use those bi-products to make something else. It is just like when you have a Thanksgiving turkey. Try to imagine all the various foods you can make out of that one turkey.

Comments: