Background Information Part 2- Nanotechnology and its applications
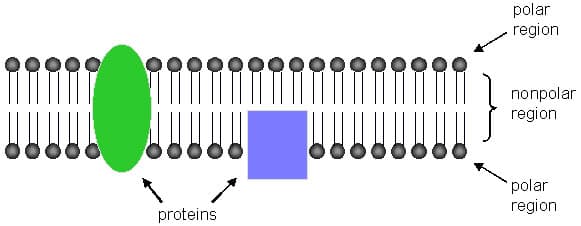
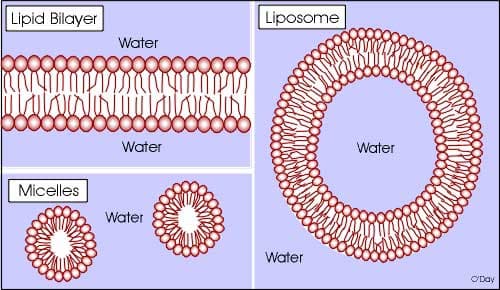
One of the key principles in nanoscience is the ability to create and design particles that are incredibly small, which can be used for a specific purpose. These particles are referred to as nanoparticles. One such nanoparticle is called a micelle. A micelle is a particle that is created by self-assembly. Self-assembly of a micelle occurs by a very similar mechanism to the biological process of formation of the fluid mosaic bilayer that makes up the plasma membrane in eukaryotic cells. The plasma membrane (diagram below) is made up of phospholipids: phospholipids are molecules that contain a phosphate group, which is hydrophilic (water-loving), and a fatty acid-lipid group, which is hydrophobic (water-fearing). When placed in water the fatty acid groups tend to assemble away from the water interface, while the phosphate group gravitates toward the water interface. A stable structure that results from these tendencies is a bilayer or—as I refer to it in my classroom, a "fat" sandwich—in which the phosphates represent the bread of the sandwich and the fatty acids represent the fatty filling. The micelle is formed in much the same way: the phosphate groups arrange so that they are in contact with the water interface and the lipids are facing away from the water interface. The shape of these structures can be altered to suit a specific purpose by varying the types or lengths of the fatty acid chains and whether or not they are generated in aqueous solution or not. (Refer to the appendix for diagramed examples). Once these micelles have been created, they can be loaded with drugs, such as cancer fighting drugs or genetic material for gene therapy.
Fluid Mosaic Model of the Plasma Membrane
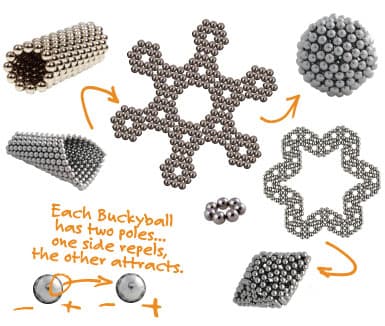
Another form of nanoparticle is called a buckyball; this is short for buckminsterfullerene (just stick with buckyball). Buckyballs are soccer-shaped structures that are made up of carbon atoms: an atom that makes up most of the structures of living organisms. These particles can also be loaded with drugs, gene therapy vectors, or other biologically active agents. Micelles and buckyballs can be dressed-up or functionalized by adding proteins, enzymes, or antigens to make the particle more likely to bond or be accepted with its designed target. Researchers have developed elaborate configurations in order to accomplish this task. This takes us into another area of science called biomimetics. Biomimetics is the science of mimicking biology or what occurs in nature. When one introduces synthetic particles into a living organism, the immune system reacts to recognize this invasion as foreign and can launch an immune attack against the particle. By encapsulating the micelle or liposome with receptor proteins or antibodies, the nanoparticle has a greater chance of entering the cells of the organism, thus delivering the intended drug or gene sequences. Biometrics is currently being used for such treatments as joint replacement therapy and tissue scaffolding.
Booker in Nanotechnology for Dummies shares, "DuPont and Exxon are using buckyballs to develop stronger polymers. These companies are looking at two ways of doing this: by integrating the buckyballs in the polymer with chemical bonding. Or by simply tossing buckyballs into the polymer and embedding them there& …buckyball-based antioxidant type drugs are being developed& hellip;for example, anti-aging or anti-wrinkle creams& hellip;and an HIV Drug." The scopes of these tools are far-reaching and have the potential to become even more so in the not too distant future. A simple search on the Internet will provide numerous articles which offer research being done in this area. Some other forms of nanoparticles include lipid colloids, ceramic nanoparticles (quantum dots), dendrimes, and nanotubles.

Comments: