Content
Overview
The growing concern about the quality of our drinking water suggests the need for accurate information about what constitutes clean water and what needs to be done to insure everyone has access to it. Water has a broad impact on health, food, energy, and economy (Savage, p xxxi). 5 In the United States, the explosion of bottled water consumption is a strong indicator of consumer distrust for municipal water (i.e. tap water). Living systems cannot survive without clean water. So, what can be done to improve the technology municipalities have to provide clean water to consumers? Let's begin with a brief discussion about the chemical properties of water.
Water Chemistry
As a chemical compound, water is simply two atoms of hydrogen joined to one oxygen atom. Though water covers our world, more than ninety seven percent is salty. Two percent is fresh water locked in snow and ice, leaving one percent for us. This "precarious molecular edge on which we survive," as Barbara Kingsolver says in the April 2010 issue of National Geographic, will only grow more precarious. The tremendous challenge and responsibility to provide clean water will become critical within the next decade in both developed and underdeveloped nations.
All of the molecular interactions in biological systems are profoundly influenced by water. Two interrelated properties of water, polarity and cohesiveness, are especially important in biological systems. Water is a polar molecule due to its triangular shape and asymmetrical distribution of charge. The oxygen nucleus attracts electrons away from the hydrogen nuclei, which leaves the region around those nuclei with an overall positive charge. The water molecule is thus an electrically polar structure. 6
Molecules of water have a high affinity for each other. They are highly cohesive. The positively charged pole in one water molecule tends to orient itself towards a negatively charged region in one of its neighbors. For example, ice has a highly regulated crystalline structure in which all potential hydrogen bonds are made. On the other hand, liquid water has a partly ordered structure in which hydrogen-bonded clusters of molecules are continually forming and breaking up. Hence, each molecule is hydrogen bonded in a ratio of 3.4 to 4.0 neighbors, in liquid water compared with ice. 6
Water is a highly interactive molecule because of its polarity and hydrogen-bonding capacity. These properties make water an excellent solvent for polar molecules. Water greatly weakens the electrostatic forces and hydrogen bonding between molecules competing for their attractions. The effects of water on hydrogen bonding between a carbonyl group (O=C) and an amide group (NH) is a noteworthy example. The hydrogen atoms of water can replace the amide hydrogen group as hydrogen donors, and the oxygen atoms of water can replace the carbonyl oxygen as the acceptor. Hence, a strong hydrogen bond between a CO and an NH group forms only if water is excluded. 6
Water has an unusually high dielectric constant because of its polarity and capacity to form oriented solvent shells around ions. These oriented solvent shells generate electric fields of their own, which oppose the fields produced by the ions. Therefore, electrostatic attractions between ions are markedly diminished by the presence of water. 6
The way water interacts with oil is interesting. When dispersed oil droplets come together in water, they typically form a single large oil drop. An analogous process occurs at the atomic level: nonpolar molecules or groups tend to huddle together in water. These associations are called hydrophobic attractions. In a figurative sense, water tends to compress nonpolar molecules together. Let us examine the basis of hydrophobic attractions, which are a major driving force in the folding of macromolecules, the binding of substrates to enzymes, and the formation of membranes that define the boundaries of cells and their internal compartments. 6
Consider the introduction of a single nonpolar molecule, such as hexane (C 6H 1 4), into some water. A cavity is created in the water, which temporarily disrupts some hydrogen bonds between the water molecules. The displaced water molecules reorient themselves to form a maximum number of new hydrogen bonds. However, this is accomplished at a cost; the number of ways of forming hydrogen bonds in the cage of water around the hexane molecule is much fewer than in pure water. The water molecules around the hexane molecule are much more arranged than elsewhere in the solution. 6
Now consider the arrangement of two hexane molecules in water. Do they sit in two small cavities or form a single larger cavity. The experimental evidence conveys that the two hexane molecules collaborate and occupy a single larger cavity. This association discharges some of the more ordered water molecules around the separated hexanes. In fact, the basis of hydrophobic attraction is this enhanced freedom of released water molecules. Nonpolar solute molecules are forced together in water because water bonds strongly to itself, not because the hexanes have a high affinity for each other. 6
Water Cycles
Water is a unique liquid; without it, life as we know it is impossible. Among the common compounds, water is the only one whose solid phase is lighter than its liquid form (it expands by about 8% when it freezes, becoming less dense). That is why ice floats. If ice were heavier than liquid water, it would sink to the bottom of the oceans, lakes, and rivers. If water froze from the bottom up, shallow seas, lakes, and rivers would freeze solid. All life in the water would die, because cells of living organisms are mostly water, and as water freezes and expands, cell membranes and walls rupture. If ice were heavier than water, the biosphere would be vastly different from the way it is, and life, if it existed at all, would be greatly altered. 7
Water is essential to life on earth. Humans can survive for more than a month without food, but we can live for only a few days without water. Two kinds of water are found on Earth: fresh water and salt water. 8 The world's water supply is located in oceans, the atmosphere, rivers and streams, groundwater, lakes, ice caps and glaciers. Ninety seven (97%) of Earth's water is in the oceans, the next largest compartment, the ice caps and glaciers, accounts for another two percent (2%). Both of these sources are generally unsuitable for human use because of salinity (sea water) and location (ice caps and glaciers). 9
Although it represents only one percent (1%), a small fraction of the water on earth, the water on land is important in moving chemicals, sculpting landscape, weathering rocks, transporting sediments, and providing our water resources. 9 Water is a renewable resource because it circulates through a water cycle in which, molecules travel between the Earth's surface and the atmosphere. 10 This hydrologic cycle transfers water from the oceans to the atmosphere (by evaporation) to land, in the form of precipitation, and back to the oceans. 11
When it rains, some of the water flows into lakes, rivers, and streams. The fresh water located in lakes, rivers, streams, and wetlands on Earth's land surface is called surface water. However, most of the precipitation percolates through the soil into regions beneath the Earth's surface. The water stored in sediment and rock formation is called groundwater. Most of the fresh water available for human use is located in this groundwater under the surface of the Earth. 12
Not surprisingly, this groundwater often contains quantities of other substances. Analyses of many wells and springs show that the elements and compounds dissolved in groundwater consist mainly of chlorides, sulfates, and bicarbonates of calcium, magnesium, sodium, and potassium. Theses substances can be traced to the common minerals in the rocks from which they were weathered. As might be expected, the composition of groundwater varies from place to place according to the kind of rock present. 13
In much of the central United States, the water is rich in calcium and magnesium bicarbonates that have been dissolved from local carbonate bedrock. Taking a bath in such water, termed hard water, can be frustrating because soap dose not lather easily and a crustlike ring forms in the tub. Hard water also leads to deposition of scaly crusts in water pipes, eventually restricting water flow. By contrast, water that contains little dissolved matter and no appreciable calcium is called soft water. With it, we can easily get a nice soapy lather in the shower. 13
Groundwater flowing through rocks that have high arsenic or lead content may dissolve these toxic elements, making it dangerous to drink. Water circulation through sulfur-rich rocks may contain dissolved hydrogen sulfide (H 2S) which, though harmless to drink, has the disagreeable odor of rotten eggs. In some arid regions, the concentration of dissolved sulfates and chlorides is so great that the groundwater is unusually noxious. 13
Surface water and groundwater interact in many ways and should be considered part of the same resource. Nearly all surface water environments, such as rivers and lakes, as well as human-constructed water environments, such as reservoirs, have strong linkages with groundwater. For example, withdrawal of surface water by diversion from streams and rivers can deplete groundwater resources or change the quality of ground water. Pollution of groundwater may result in pollution of surface water, and vice versa. 13
Water Quality
Clean water is a resource that we often take for granted. We expect a colorless, odorless, and tasteless liquid to come from our faucets on every turn. More than one billion people in the world lack access to clean water, and the severity of the problem increases with time. 14 A clean water supply is essential to our health, economy, and way of life. The distribution and purification of this vital resource is dependent upon a reliable infrastructure and proficient water treatment system.
Clean Water Act
In 1969, the Cuyahoga River in Cleveland, Ohio, was so polluted that the river caught on fire and burned for several days. This shocking event was a major factor in the passage of the Clean Water Act of 1972. The stated purpose of the act was to "restore and maintain the chemical, physical, and biological integrity of the nation's water." The goal of the act was to make all surface water clean enough for drinking and swimming by 1983.
The Clean Water Act opened the door for other water-quality legislation. In 1972, the Marine Protection, Research, and Sanctuaries Act was also passed. This act empowered the Environmental Protection Agency (EPA) to control the dumping of sewage, wastes, and toxic chemicals in U.S. waters. Other laws were passed during this period to improve water quality in the United States: the Safe Drinking Water Act (1975), which introduced programs to protect groundwater and surface water from pollution; the Comprehensive Environmental Response Compensation and Liability Act (1980); the Water Quality Act (1982); and the Oil Pollution Act (1990), which could be amended in the near future because of the devastation caused by the BP oil spill (2010) in the Gulf of Mexico.
Water Pollution
Water pollution is the introduction of a biological, chemical, or physical substance that, in an identifiable excess, is known to be harmful to living organisms that depend on the water source for survival. Water pollution is caused by the sudden or continuing, accidental or deliberate discharge of contaminating material(s). Water is most often polluted by sewage, extracted fossil fuels, burned fossil fuels, oil spills, fertilizers, pesticides, erosion, and by-products of manufacturing. Various toxic chemicals have been found in water (see Table 1):
| Compound | Toxic Levels (ppm) |
| Trihalomethane | 0.10 |
| Nitrate | 10.0 |
| Nitrite | 1.0 |
| Silver | 0.05 |
| Cadmium | 0.005 |
| Mercury | 0.002 |
Table 1: Toxic chemicals that are often found in water, with concentrations that are known to be toxic to humans. Source: Botkin and Keller, 2005.
In addition to heavy metals, water pollutants include sediment, certain radioactive isotopes, heat, fecal coliform bacteria, phosphorous, nitrogen, sodium, and prescription drugs, as well as certain pathogenic bacteria and viruses. Today, the primary water pollution problem in the world is the lack of clean, disease-free drinking water. More than one-quarter of drinking water systems in the United States have reported at least one violation of federal health standards. 15
The U.S. Environmental Protection Agency (EPA) has set thresholds, or limits, on water pollution levels for some (but not all) pollutants. As a result of difficulties in determining effects of exposure to low levels of pollutants, maximum concentration standards have been set for only a small fraction of the more than 700 identified drinking water contaminants. 15
The traditional foe of water quality is waste from factories and farms, but now environmental regulators are eyeing a new pollution source: our medicine cabinets. Baylor University researchers found norfluoxetine, carbamazepine, diphenhydramine, and dialtiazem in fish pulled from Chicago's North Shore: Fish caught downstream from sewage treatment plants in five U.S. cities contained traces of pharmaceuticals and toiletries. To assess the risk, the EPA has expanded monitoring to 150 sites, with results due in 2011. 16
Water Toxicology
Small amounts of pollutants or toxins in the environment, such as pesticides are reported in units as parts per million (ppm) or parts per billion (ppb). It is important to keep in mind that this unit can mean different things; the ppm or ppb concentration may relate to either volume, mass, or weight. In some toxicology studies, the units used are milligrams of toxin per kilogram of body mass (1 mg/kg is equal to 1 ppm by mass). Concentration may also be recorded as percent. For example, 100 ppm by mass (100mg/kg) is equal to 0.01%. 17
When dealing with water pollution, units of concentration for a pollutant may reported in milligrams per liter (mg/L) or micrograms per liter (ug/L). A milligram is one-thousandth of a gram, and a microgram is one millionth of a gram. For water pollutants that do not a cause significant change in the density of water (1g/cm 3), a concentration of pollution of 1 mg/L is approximately equivalent to 1 ppm by mass. 17
Water Treatment
There are thousands of potentially harmful substances in our drinking water in even smaller concentrations than ppm or ppb, and current methods of chemical analysis and toxicity testing cannot determine the long term effects of small doses ingested every day. The challenges facing water purification facilities in the United States are multifaceted; there is a greater diversity of chemicals in the water at smaller concentrations, requiring more efficient, cost-effective, and reliable treatment technologies. 18 Quality standards for drinking water and water treatment methods must be adapted to meet current contaminant levels. 19
Water for domestic use must be free from constituents harmful to health, such as insecticides, pesticides, pathogens, and heavy metal concentrations. It should taste good, should be odorless, and should not damage plumbing or household appliances. In urban areas, water purification occurs at specially designed facilities that accept municipal sewage form homes, businesses, and industrial sites. The raw sewage is delivered to the plant through a network of sewer pipes. Following treatment, the wastewater is discharged into the surface water environment (river, lake, or ocean) and recycled to homes and businesses, or in some limited cases, used for another purpose such as crop irrigation. 20
The main purpose of standard treatment plants is kill bacteria and other pathogens with chlorine and UV radiation, and to remove toxic chemicals through sedimentation and filtration methods. Wastewater treatment methods are usually divided into three distinct categories: primary treatment, secondary treatment, and advanced treatment. Primary and secondary treatment are required by federal law for all municipal plants in the United States. 20
How Safe Is Our Drinking Water?
In 1993, 400,000 people in Milwaukee got sick from Cryptosporidium contaminated tap water and 104 citizens died. Cryptosporidium is a one-celled parasite that can cause a gastrointestinal illness called cryptosporidiosis. The symptoms are acute and chronic cases of diarrhea, abdominal cramps, fever, and vomiting. Since the microorganism is only 5 microns in length, 250 of them can fit on the head of a pin. They can slip through typical water treatment plants and enter drinking water. 21
The highly resistant cyst of Cryptosporidium parvum allows the pathogen to survive various drinking water filtrations and chemical treatments such as chlorination. Although municipal drinking water utilities may meet federal standards for safety and quality of drinking water, complete protection from cryptosporidial infection is not guaranteed. In fact, all waterborne outbreaks of cryptosporidiosis have occurred in communities where the local utilities met all state and federal drinking water standards. 22
Even though most municipal water supplies are treated according to the Safe Drinking Water Act, millions of Americans are still wary of drinking tap water. More significant than the threat of bacterial contamination, is the growing concern over chemical pollutants. There are thousands of chemical compounds in our water. They originate from industry, agriculture, and consumer homes. Small doses of these chemical combinations are ingested every day; yet, we do not know what their long term effects will be.
Landfills are a major source of groundwater contamination. Industrial waste and municipal sewage continue to make their way into our waterways. Cesspools, septic systems, underground storage tanks, and lawn treatments (pesticides and fertilizers) all runoff into our water. Even toxic gasoline additives such as MTBE, seeps into our ground water due to inadequate storage procedures. As if contamination from gasoline, parasites, pesticides, and fertilizers were not enough; aging pipes, heavy metals, suspected carcinogens and even radioactive waste have an impact on our water. 22
Obviously, there are numerous reasons to be concerned about the safety of tap water. What are some steps we can take to make sure our drinking water is safe? First, we can familiarize ourselves with the Department of Public Utilities (DPU) Annual Water Quality Report to evaluate our water safety record. Second, we can investigate the effectiveness of various in home water treatment methods, from boiling water to sophisticated filtration and distillations systems. The most common alternative is to drink bottled water. Yet, this option raises some challenging questions for consumers.
Every second of every day in the United States, a thousand people buy and open up a plastic bottle of commercially produced water, and every second of every day in America, a thousand plastic bottles are thrown away. How many bottles do Americans discard every day (see appendix)? More than thirty billion bottles a year are thrown away at an enormous cost to consumers. And for every bottled consumed in the U.S., another four are consumed around the world. 23
Why do we buy bottled water? Is it as safe as tap water, or even safer, as we are often told? What are the environmental and social consequences of bottled water use for the planet? The beverage industry tells us that bottled water is just a simple commodity like any other food product, a safe, well-regulated alternative to tap water. The environmental community tells us bottled water is a corporate conspiracy to privatize a precious public resource and that it's less safe than our tap water. 23 What is the truth? More importantly, what can we do as a global society to insure that all living organisms have access to a reliable supply of clean drinking water?
Nanotechnology: Applications for Clean Water
Access to clean water is a bigger problem for exploited and under industrialized areas than hunger. Within the next two decades, the average supply of water per person will drop by one third, possibly condemning millions of people to severe dehydration and avoidable premature death. 24The design and manipulation of atomic and molecular scale (nanoscale) materials offers great possibilities for advances in cleaner energy production, energy efficiency, and water treatment. 25
Iron Remediation
Pioneering research by environmental engineer Wei-xian Zhang of LeHigh University in Pennsylvania, has shown the potential of iron nanoscale powder that is able to clean up soil and groundwater previously contaminated by industrial pollution. Iron is one of the most abundant metals on Earth and it just might be the solution to a trillion-dollar problem stemming from thousands of contaminated sites in the United States. 25
The answer seems to come from the fact that iron oxidizes around contaminants such as trichloroethylene, carbon tetrachloride, dioxins, or PCBs. These organic molecules are broken down into simple, far less toxic carbon compounds. Similarly, with toxic heavy metals such as lead, nickel, mercury, or even uranium, oxidizing iron reduces them to an insoluble form that is locked with the soil, rather than mobilized and available to leach into groundwater. 25
Nanoscale iron particles are 10 to 1000 times more reactive than commonly used iron powders. Smaller size also gives nano-iron a much larger surface area, allowing it to be mixed into a slurry and pumped straight into the center of a contaminated site, like a giant IV injection. Upon arrival, the particles flow along with the groundwater, decontaminating the environment as they go. 25 The iron nanoparticles are small enough that water can carry them into the soil that the water is contaminating. When they react with oxygen in the water, the iron nanoparticles oxidize into rust — a reaction that helps neutralize contaminants that come in contact with the particles. 26
Iron particles are not changed by soil acidity, temperature, or nutrient levels. Their size (1-100 nm in diameter and 10-1000 times smaller than most bacteria) allows them to move between soil particles. Laboratory and field studies have shown that nanoscale iron particles treatment drops contaminant levels around the injection well within a day or two and nearly eliminates them with a few weeks, bringing the treated area back into compliance with federal groundwater quality standards. Results have also indicated that the nanoscale iron stays active in the soil for six to eight weeks before the nanoscale particles become dispersed completely in the groundwater and become less concentrated than naturally occurring iron. 27
The quality and effectiveness of iron nanoparticles may vary depending on the lab that makes them. Different batches may produce deleterious results. For example, one showed that in combination with CCl 4 (carbon tetrachloride), nanoparticles coated with an iron oxide containing boron turn CCl 4 into chloroform which is poisonous. On the other hand, nanoparticles coated with an iron oxide containing sulfur turn CCl 4 into harmless molecules. 28
Center for Biological and Environmental Nanotechnology (CBEN) researchers at Rice University have developed a reactive membrane from iron oxide ceramic membranes (ferroxanes). Due to iron's unique chemistry, these reactive membranes provide a platform for removing contaminants and organic waste from water. Ferroxane materials have even been found to decompose the contaminant benzoic acid. 28
Nanofluidic Carbon Nanotube Membranes: Applications for Water Purification and Desalination
Membranes and filters of all sizes are used to separate various substances. For example, coffee filters remove coffee grains resulting in a macro particle free cup of coffee and non specific laboratory filters allow students to separate NaCl from saline solutions. In an demonstration of osmosis, a selectively permeable membrane permits water to diffuse into a more concentrated solution.
The carbon nanotube (CNT) has firmly established itself as the iconic molecule of nanoscience. A CNT is simply a nano-meter sized rolled-up atomically smooth grapheme sheet that forms a perfect seamless cylinder capped at the ends by fullerene caps. It is common to characterize the structure of the nanotube by its rolled-up vector, called chirality or helicity, which defines the position of the matched carbon rings during the roll-up of the grapheme sheet. A CNT can have one (as in the case of a single-walled CNT), or several concentric graphite shells (as in the case of multi-walled nanotubes) and it can reach up to several millimeters in length, yet retain a diameter of only a few nanometers. 29
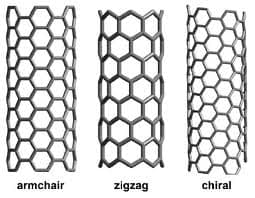
Figure 1: Configurations (i.e. armchair, zigzag, and chiral) of the carbon nanotube. 30
Several methods of CNT production currently exist. In the laboratory environment, catalytic chemical vapor deposition (CVD) is preferred over other methods such as arc discharge and laser ablation because it produces higher quality CNTs. CVD reactors can produce individual isolated nanotubes, as well as densely packed vertically aligned arrays. Unfortunately, the ultimate goal of the CNT synthesis—producing a uniform population of nanotubes with a given chirality—still remains elusive. Several studies indicated that the size of the catalyst particle during the growth stage determines the size of the CNT to less than 10 percent; yet efforts to control the size of the CNTs with greater precision have been largely unsuccessful. Thus, synthesizing a vertically aligned CNT array with narrow distribution of sizes still remains a difficult endeavor requiring considerable process development and optimization efforts. 31
Nanotechnology promises to dramatically enhance many water purification technologies However, one of the key issues related to nanotechnology is the question of how to apply it. Specifically, it is not clear how to interface nanoparticles with contaminants. At present, many expensive nanoparticles cannot be added to water like commodity chemicals and some nanoparticles could present new hazards to human health and the environment. 31

Comments: