Background
Hair: A natural polymer
Essentially, the biology of hair is the same, regardless of ethnicity. Hair is a biopolymer that is "90% made out of a hard, fibrous protein called keratin. Keratin is made up of polypeptide chains of amino acids such as glycine, alanine, and cysteine. Polypeptide bonds hold these individual amino acids together and there are multiple other complex bonds involved. 3 Taken together, the polypeptide chain that makes up human hair is an alpha helix. In one single strand of hair, three alpha helices are twisted together to form a protofibril. Protofibrils are the initial elongated unit appearing when any type of fiber is being formed. Nine protofibrils will join in a circle around two or more to form an 11-stranded cable that is called a microfibril. Hundreds of these microfibrils will cement into an irregular fibrous bundle called a macrofibril. These macrofibrils are then joined to make the cortex or main body of the hair fiber. 4
"Keratin has three main morphological areas, the cuticle or translucent scale like outer layer of the hair shaft, the cortex which can be conceptualized as the meat of the hair shaft, and the medulla which is the central core of the hair shaft." 5 "It is the shape of the hair follicle that directly influences the shape of the hair shaft and accounts for human genetic variation in texture. According to forensic scientists, Douglas Deedrick and Sandra Koch, "the morphology of hair by race reveal that Caucasian hair is oval to round shaped with even pigment and a medium size cuticle." Deedrick writes, "that it is this oval shape that gives the Caucasian hair a straight or mildly wavy appearance. For identification purposes, round hair shafts with an auburn pigment and thick cuticle are sorted into the category of Mongoloid or Asian hair. "This shape of the hair cells," he notes, "makes it appear very straight." For Negroid or African hair he explains, "hair shaft has been categorized as appearing to be primarily elliptical or flat shaped with dense pigmentation and a thin cuticle. The flat shape of the hair cells combined with naturally lower levels of scalp oil and larger amounts of sulfur gives African typed hair an extremely curly appearance. This texture of hair is susceptible to twisting or buckling, conditions that can create splits in the hair shaft. These factors work in unison to create the tightly twisted hair that is associated with people of African descent." 6
The versatility of African American hairstyles presents a wealth of insight into the social and cultural identities of African American people. Additionally, the hair types and fashions of non-African Americans can inform awareness of those cultures, as well. Pointedly, the varied possibilities of hairstyles for any ethnic group rest on the physiology of hair and hair textures. However, with the inception of polymers in our world, the considerations of hair texture to hairstyles has lost its merit. All types of artificial/synthetic hair products are available in the market to address styling needs and draw down genetic encumbrances to hair changeability.
Artificial Hair: A synthetic polymer
Synthetic hair like human hair is a polymer. It is composed of fine plastic fibers, manufactured to look like human hair. In its basic form, synthetic hair is made from low-grade acrylic that is heated and strung into strands to make individual hair fibers. The strands are then laced or tied into extensions and hairpieces. Typically, these types of synthetic hair fibers are used in costume wigs, and lack the movement and texture of real hair. Additionally, the appearance of these fibers is waxy and plastic. Conversely, the more sophisticated form of synthetic hair has texture and luster, and resembles human hair. Manufacturers of this artificial hair make use of polymer science to create these hair fibers. The fibers range in type from straight to kinky/curly with coarse surfaces to silky/smooth surfaces. The complex methods of spinning synthetic polymers into fibers include using volatile solvents and a spinneret to form multiple continuous filaments. Both, monofilament fibers and polyfilament fibers are used to fabricate the hair. Typically, fibers of modacrylic, vinyl chloride, vinylidene chloride, polyester, nylon are used for artificial hair. 7 Each of these fiber types are made by combining two or more polymers in one chain via melt spin or solvent spin processes. Rarely is one type of monofilament used. By linking the two polymers together, manufacturers of synthetic hair are attempting to develop a product that would more closely capture specific properties of natural hairs. For example, the synthetic hair fiber, modacrylic is one chain with segments of two different polymers, vinyl chloride and acrylonitrile. Modacrylic fibers have been used conventionally to make wigs, false hair, hair bands and doll hair. 7 The synthesis of modacrylic fibers involved utilizing the properties of each polymer to graft out a suitable product. In this case, the intended product would be flame resistant, have high tensile strength, and appropriate refraction properties. 7 The chemical processes for making this kind of synthetic hair fiber and others are varied and can be quite complicated to understand, without a basic knowledge of polymer chemistry.
What is a Polymer?
Certainly, a polymer is more than a hair fiber. However, a fiber is a good entry point for understanding the science of polymers. A polymer, whether natural or artificial, is a type of macromolecule. Macromolecules are substances with large molecular masses and hundreds to thousands of atoms chemically combined to each other. Polymers are enormous substances, made up of molecules with distinct structural features that repeat themselves, again and again. Each repeating molecular structure within a polymer is called a monomer (mer). An example of a monomer is ethylene. Ethylene, a gaseous organic compound, has a chemical formula of CH 2CH 2. As a polymer, the molecules of ethylene would chemically combine to form the structure below:
-CH 2CH 2-CH 2CH 2-CH 2CH 2-CH 2CH 2-CH 2CH 2CH 2CH 2-CH 2CH 2-CH 2CH 2-CH 2CH 2- , e t c
This long chain would go on and on, hundreds of monomers (mers) long until terminated by chemical processes. The name of this polymer is polyethylene, because of the many mers of ethylene. Polyethylene is commonly used in water bottles, garbage bags, ballistic wear, textile fibers and automotive parts. Other elements common to monomers are oxygen, nitrogen and the halogen elements. To illustrate this point, consider the monomer, vinyl alcohol, CH 2=CH-OH, combining to form the polymer, polyvinyl alcohol below:
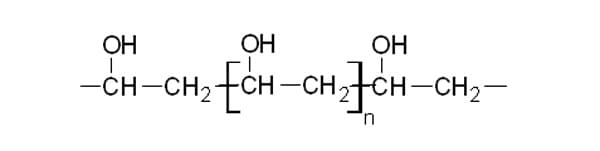
Polyvinyl alcohol has many usages. It is used as a binder and thickener in paper cloth, films, cements and mortars. In the food and cosmetic industry, polyvinyl alcohol protects the active ingredients in products from moisture, oxygen and other environmental components, while preserving the taste and odor. 9 Like most, polymers, polyethylene and polyvinyl monomers are made of the elements, carbon and hydrogen (also known as hydrocarbons). Many macromolecules are derived from this class of substances called alkanes. Alkanes are hydrocarbons that contain only single bonds. If the bonds between the carbon and hydrogen become doubled, an alkene is formed.
What is an alkene?
As stated, alkenes are hydrocarbons that contain double covalent bonds. They can be linear or cyclic in structure. The simplest alkene is ethene; it has two carbon atoms and four hydrogen atoms. The general formula for linear alkenes with one double bond is C nH 2 n, where n equals the number of carbon atoms. The carbon atoms linked by double bonds are not combined with as many atoms as those linked by only single bonds. This special feature of an alkene is central to the chemical reactivity of these molecules. Many reactions of alkenes involve the carbon-carbon double bonds. Primarily through addition chemistry, alkenes can react to form alcohols, to form halogen compounds and to become hydrogenated to form alkanes. The carbon-carbon double bonds are broken and single bonds formed with added substances. Because alkene structures are easily manipulated to form new molecules through addition reactions, a variety of polymers can be formed from this class of molecules.
Chemical Bonds
The bonds holding these macromolecules together are covalent bonds. The covalent bonds are the primary bonds of polymer molecules. These bonds are strong, directional and exist between the carbon atoms in the chain of these molecules. In addition to these bonds, there are weaker bonds between the chains called secondary bonds. Specifically, these bond types are hydrogen bonds and van der Waals bonds. Hydrogen bonds can exist between molecules of long chain polymers. They are stronger than van der Waal forces and can be used to strengthen the structure of a polymer. Like covalent bonds, they are directional; that is they point in the direction of the hydrogen atoms in the molecule. This characteristic of hydrogen bonding can account for the low density of long polymers. Van der Waals forces are the weakest of all intermolecular interactions. They are the result of electrostatic attraction between polar molecules or molecules with fluctuating dipoles and are non-directional. "Van der Waal forces exist between long polymers, as well. The presence of this weak force, between these molecules, accounts for the easy movement of the long polymer chain. This easy movement of the chains is what causes many polymers to be plastic or easily deformed." 8
Polymer Structure
Polymers can be linear, branched or cross linked. Linear polymers are free to move. They slide back and forth against each other easily, particularly when heated. Branched polymers contain side chains that prevent the molecules from sliding across each other easily. In cross-linked polymers, adjacent molecules in the polymers have formed bonds with each other. As a result, the individual molecules are not able to slide past each other when heated. Three methods can affect the structure and strength of a polymer. First, the introduction of atoms, such as oxygen and sulfur between chains of polymers, can inhibit their sliding over each other. This method is called crosslinking. A second method called crystallization would put chains of polymers close together over long distance. This would increase the strength of the interactions between the molecules and, thus, strengthen the polymer. The third method requires adding a large side group to the chain. This would stop bonds from rotating and also congeal the chain, making it more difficult for the macromolecules to move past each other. 8
Homopolymers and Copolymers
Polymers can be characterized as homopolymers or copolymers. Both, polyethylene and polyvinyl alcohol are homopolymers. The term homopolymer is used to describe polymers whose structure can be represented by the repetition of a single type of repeating unit containing one or more molecules. 10 If given a hypothetical monomer "A", the homopolymer would be:
etc.-A-A-A-A-A-A-A-A-A-A-A-A-A-A-A-A-A-A-A-A-etc.
Or, if the species represented is a monomer "AB", then the homopolymer would be:
etc.-AB-AB-AB-AB-AB-AB-AB-AB-AB-AB-AB-AB-etc.
Sometimes two monomers can combine and polymerize, providing a unit "a" and a unit "b". When this occurs, a copolymer has formed. "The formal definition of a copolymer is a polymer derived from more than one species of monomer." 10 Copolymerization, in its objective, is very useful for synthesizing a polymer with the required combination of properties. Essentially, the properties of two or more monomer species are combined to create a polymer material that takes advantage of the monomers' individual characteristics. In the development of synthetic hair, this is an important process. Chemist and manufacturers of hair fibers utilize this technique to control the properties of the monomers in order to graft out hair materials meeting certain product specifications, e.g. tensile strength, combing property, and heat resistance.
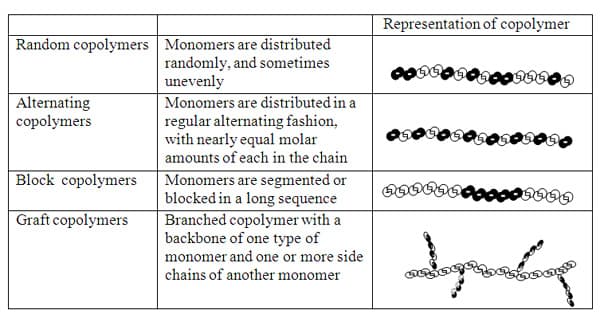
There are four copolymer structures as shown and defined in Table 1 below.
Table 1. (refer8, 10,11) Types of copolymers with characterization.
Styrene butadiene rubber is an example of a block copolymer. It is composed of unit a- H 2C=CHC 6H 5, styrene, and unit b-H 2C=CH-CH=CH 2, butadiene. As defined, this type of copolymer is linear with a long sequence or block of one monomer joined to a second monomer. This macromolecule would have the following units in its structure:
etc.-a-a-a-a-a-b-b-b-b-b-b-a-a-a-a-a-b-b-b-b-b-b-etc.
Specifically, the styrene butadiene rubber (SBS) is made up of three segments – an initial long chain of polystyrene, a long chain of polybutadiene and another long section of polystyrene.
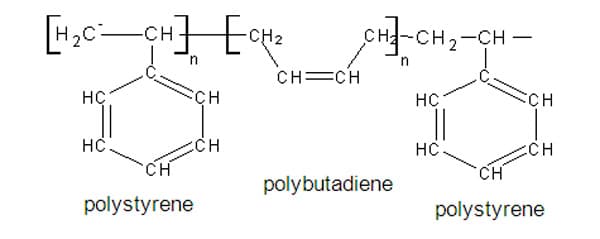
Polystyrene is a hard, resilient, transparent polymer, while butadiene is tough and hard, but brittle. Together, styrene butadiene rubber (SBS) is a hard rubber, used to make the sole of shoes, tire treads, other durable items. In combining the properties of these polymers, the resulting copolymer, SBS is more impact resistant and durable. 12
PolymerizationBlock copolymers, like other synthetic polymers do not occur naturally. The reaction that makes these polymers and others is polymerization. In general, there are two types of polymerization mechanisms, addition (chain reaction) and condensation (step reaction) polymerization. Condensation polymerization begins with bifunctional monomers. Monomers with two sites for bonding are bifunctional. Functionality is a very useful concept in polymer science. The functionality of a molecule is the number of sites it has for bonding to other molecules under the given conditions of the polymerization reaction. 10 Therefore, bifunctional monomers must have chemically active bonding sites where another molecule can link to build the polymer. The minimum functionality required for polymerization is two. These bonding sites are on the ends of the monomer. The reactions that develops at the sites are relatively slow and begins when the monomers of each molecule react to bond covalently. In condensation polymerization, the molecules are linked together by the removal of a small molecule, such as water or acid. For example, those monomers having functional groups, such as amines and carboxylic acids, can react with each other, generally grow by carbon-heteroatom bond formation (C-O and C-N), producing water as they grow. To illustrate, the condensation reaction for the synthesis of Dacron and Nylon-6,6 shows the formation of water as the polymers grow.
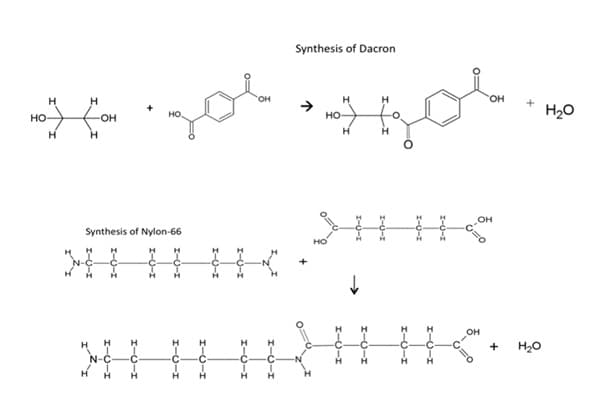
As presented, Dacron, a type of polyester fiber, is the result of a condensation reaction. In this example, the polyester is created using two monomers, both bifunctional, dicarboxylic acids (two carboxyl groups on each end) and diols (two alcohol groups on each end). The products are again the macromolecule, polyester and water. Similarly, nylon, a type of polyamide is formed when diacids (adipic acid) react with diamines (hexamethylene diamine) to polymerize and yield water. Figure 1, displays a corresponding reaction with bifunctional polyamides (two amines on each end) and bifunctional dicarboxylic acids (two carboxyl groups on each end). 10 ,12
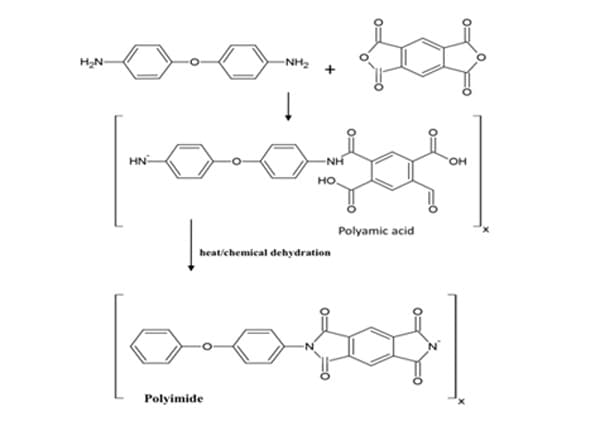
Figure 1
Examples of natural condensation polymers include wool, silk and cellulose. Some synthetic condensation polymers are polyamide, polyester, polyurethane, polysulphide and polysiloxane.
The addition polymerization process or chain reaction begins with a low molecular weight monomer with double bonds, such as ethylene, CH 2 = CH 2. Unlike the condensation process, the addition reaction results in no byproducts. Polymers formed by this mechanization do so by adding, in succession, unsaturated monomers in a chain reaction. The process involves the treatment of the double bonded monomers (alkenes) with an initiator substance to open up the double bond to release a free radical and valence electrons. Subsequently, these free electrons will join with other molecules in the reaction to form a polymer chain. For example, ethylene polymerizes to polyethylene:
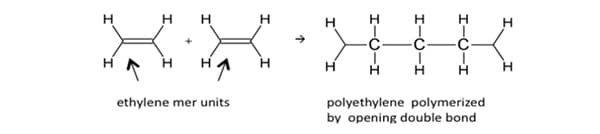
In this reaction, no side products are formed and the composition of the mer or repeating unit of the polymer –CH 2-CH 2-CH 2-, is identical to that of the monomer CH 2=CH 2. These are important features of the chain polymerization process. Chain polymerization involves three processes: chain initiation, chain propagation, and chain termination. Chain initiation occurs by an attack on the monomer molecule by a free radical, a cation or an anion; accordingly, the chain polymerization processes are called free radical polymerization, cationic polymerization or anionic polymerization. A free radical is a reactive substance having an unpaired electron and is usually formed by decomposition of a relatively unstable material called an initiator. Organic peroxides, like benzoyl peroxide are common free-radical initiators. The free radical, in general, is very active because of the presence of unpaired electrons. Free radical species can, thus, react to open the double bonds of a monomer, and add to one side of the broken bond, with the reactive center (unpaired electron) being transferred to the other side of the broken bond. 10 At this point a new species is produced. It will react to attack a second monomer molecule, transferring its reactive center to the attacked molecule. This process repeats, and the chain grows as hundreds of monomer molecules are sequentially added to propagate (chain propagation) the reactive center. Interference of the propagation process by termination reactions (chain termination) destroys the reactive center, ending the growth of the polymer. 10 The subsequent polymer is extremely large and would have propagated in a short time, usually a few seconds or less. The polymer, styrene butadiene rubber (SBS), is the resultant product of an anionic vinyl polymerization. 12 The chemical mechanization is shown below:
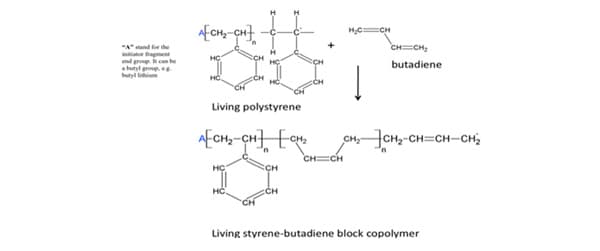
Other examples of addition polymers include low-density propylene, polyacrylonitrile, polyvinyl acetate, and polyvinyl chloride.
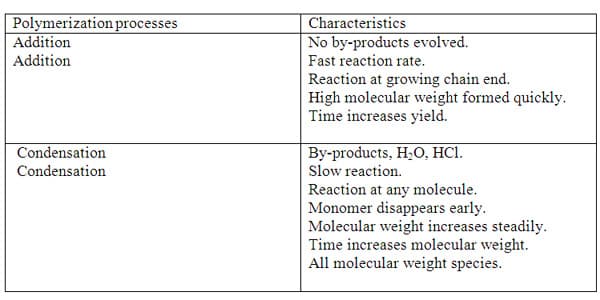
Some important differences of each polymerization processes are summarized in Table 2.
Table 2.
Synthetic hair fibers – copolymerization
Synthetic hair fibers are copolymers. Whether addition or condensation polymers, these fibers in their design are a mix of polymeric properties characteristic of the polymers used in the grafting. Created by a process of extruding polymer materials through spinnerets, in order to form microfilaments or fine thread, most synthetic hair fibers, are a blend of the common polymers – nylon, polyester, and acrylic. Research of recent U.S., European and Japanese patents, reveal that the monomers used most often in synthesizing hair fibers were polyvinyl chloride, acrylonitrile, and polyester. For example, the intended fibers described in U.S. patent 7906209B2 would exhibit properties of bulkiness, touchable (soft) texture, dyeability, settability and gloss. To obtain synthetic fibers of these qualities, manufacturer proposed combining monomers, vinyl chloride and acrylonitrile in specified proportions (by percentage weight) and geometries. 13 Similarly, the idea of the European patent 0355749B1 was to develop copolymer fibers "comprising 70-85% by weight of repeating unit, vinyl chloride and 15-30% by weight of acrylonitrile." With the idea of producing artificial hair that mimics the compatibility and texture of natural hair, manufacturers employed the resilience and spiraling (curl) properties of vinyl chloride, as well as the bulky, textural appeal of acrylonitrile in designing their fiber. 14 In the U.S. Patent 3727619, a method for joining synthetic hair and human hair together, using ultrasonic vibrational energy is prescribed. Copolymerization of synthetic fibers with human hair fibers would be achieved by overlapping portions of both fibers at fixed positions to each other. The "ultrasonic vibrational mechanical energy would be introduced into the area of overlap in a proper direction and for a period of time to obtain a bond between the overlapping segments of hair." 15 Though, the science behind the design and manufacture of synthetic hair fibers is experimental and evolving, polymers are a mainstay in our society. They are part of our natural and industrial world. Thus, the science principles behind the everyday materials in our lives involve our understanding polymers – their properties and behavior.

Comments: