Hair Treatment
Permanent Waves
Turning straight hair to curly is a matter of breaking the cross linkages in keratin. This can be done by using a permanent wave solution. A typical permanent wave solution contains 7% ammonium thioglycolate, 3% ammonium hydroxide and water. 9
Hair is first dampened so that water can break the hydrogen bonds between keratin chains. When the permanent wave solution is applied, the alkali in the solution, ammonium hydroxide, breaks the ionic/salt bridges. The –OH of the ammonium hydroxide reacts with the positively charged amino group (—NH 3 +) . The amino group then loses its positive charge. Its attraction for a neighboring negatively charged carboxyl ion dissipates and the ionic bond between the ionized amino and carboxyl groups collapses.
The only bonds left in the cross linkages are the very tough disulfide bonds. The base in the permanent wave solution is not even strong enough to break the disulfide bonds. A redox reaction does the job.
Ammonium thioglycolate serves as a reducing agent. It adds hydrogen to the sulfur atoms that make up the disulfide bond. This is the reduction part of the reaction. This breaks the bond between the two S atoms.
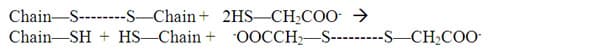
With the three bonds forming the cross linkages broken, the hair can now be reconfigured. Sections of the hair are carefully coiled around rollers. Hair readily takes the shape of the curlers because the cross linkages have collapsed.
At this point, oxidation, the other half of the reaction, is ready to be carried out. After the hair is rinsed with water to remove the excess ammonium thioglycolate, hydrogen peroxide, which hairdressers call a neutralizing agent but is really an oxidizing agent, is applied. The oxygen of hydrogen peroxide removes the hydrogen atoms that were added to the sulfur atoms of the cysteine residues, forming water. The sulfur atoms, now free of hydrogen, connect with each other, to form disulfide bonds. These new disulfide bonds lock the keratin chains in the new "curly" position.
2e - + H 2O 2 + 2H + -> 2H 2O
The permanent wave solution has a pH ranging from 8 to 9.5. The high pH opens up the cuticle, allowing the chemicals to go in and react with the keratin faster.
The base in the permanent wave solution can be replaced by an acid but it does not open up the cuticle so the process takes longer.
Hair Straightening
Hair straightening can be done using heat or chemicals. Heat from a flat iron (with a temperature range between 170 0C - 230 0C) breaks some of the hydrogen bonds that comprise the cross linkages in keratin. 10 Brushing the hair then stretches the protein strands and new hydrogen bonds form in new positions in the now straighter hair. This is, however, a temporary configuration. Water can easily break these hydrogen bonds and the protein strands go back to their old configuration.
Chemical straightening is a more permanent way of having one's hair straightened. The principle is the same as getting a permanent wave. The three bonds that form the cross linkages need to be broken so that hair can be reshaped.
One of the more common techniques uses Japanese thermal straighteners. Just like permanent wave solutions, one of its active chemicals is ammonium thioglycolate which, as described above, breaks disulfide bonds in a reduction reaction. A flat iron is then used to reshape the keratin strands into a straighter configuration after which an oxidizing agent like hydrogen peroxide or sodium bromate establishes new disulfide bonds in new sites that will keep the strands straighter.
Another technique called the Brazilian blow-out involves adding amino acids from keratin to the protein fiber in hair. A cross-linking reagent is used to stick the extra amino acids to the keratin strands in hair after which the hair is stretched and heated with a flat iron. This technique has generated controversy because one of the cross-linking reagents used is formalin, which is considered a carcinogen when inhaled. 11
Hair Coloring
A conventional permanent hair dye solution contains hydrogen peroxide, ammonia, dye precursors and a surfactant. The hydrogen peroxide and ammonia react to form perhydroxyl ion and an HO radical which raise the pH of the solution between 10-11. The very high pH lifts and opens the scales of the cuticle as the hair fiber swells. It is necessary to open up the cuticle because the molecules that make up the dye precursors are too large to go through the cuticle. Once the cuticle opens up, the dye precursors are able to go in and reach the cortex.
The perhydroxyl ion has two functions. It oxidizes the dye precursors which enable them to fuse together to form the desired color. At the same time, it reacts with the melanin, bleaching it in the process. The surfactants keep the hydrogen peroxide and ammonia dissolved in the solution and make the chemicals stick to the hair while the treatment is being done.
The last step involves the use of an appropriate shampoo or cream rinse to close up the scales of the cuticle, trapping the new color inside the cortex. This allows the hair to keep its color for a long time. The color is not washed off by shampoo.
Though the term used is permanent hair coloring, the color does not stay permanent. The new hair that grows from its root will have the original color.
One trade off of using this combination of permanent hair dye solution is the damage the hair suffers from the high alkalinity of the solution. To lessen hair damage due to high pH, chemists from Proctor and Gamble came up with a formula that lowers the pH and eliminates the formation of the HO radical which causes a lot of hair damage.
In 2008, the company launched a new line of permanent hair dyes that uses ammonium carbonate, hydrogen peroxide, and the amino acid sodium glycinate. The reaction produced a new set of reactive species: a peroxymonocarbonate ion (HCO 4 -) and a carbonate radical (CO 3 2-). The peroxymonocarbonate ion permits the bleaching and coloring to occur at pH 9, causing less damage to the hair. Further damage is reduced by the addition of sodium glycinate which removes the carbonate radicals as they are formed. 12
Semi-permanent hair coloring involves the use of a colorant and not a dye precursor so there is no oxidation involved. The colorant's pigment molecules are small enough to penetrate the cuticle and settle around the cortex but they do not react with the melanin. The color gradually fades and may be gone after 5-10 shampoos.
Temporary hair coloring also uses a colorant. Because the colorant molecules are large, they cannot go through the cuticle. Instead they just coat the cuticle and the color washes out after 2-3 shampoos.

Comments: