Appendix 2
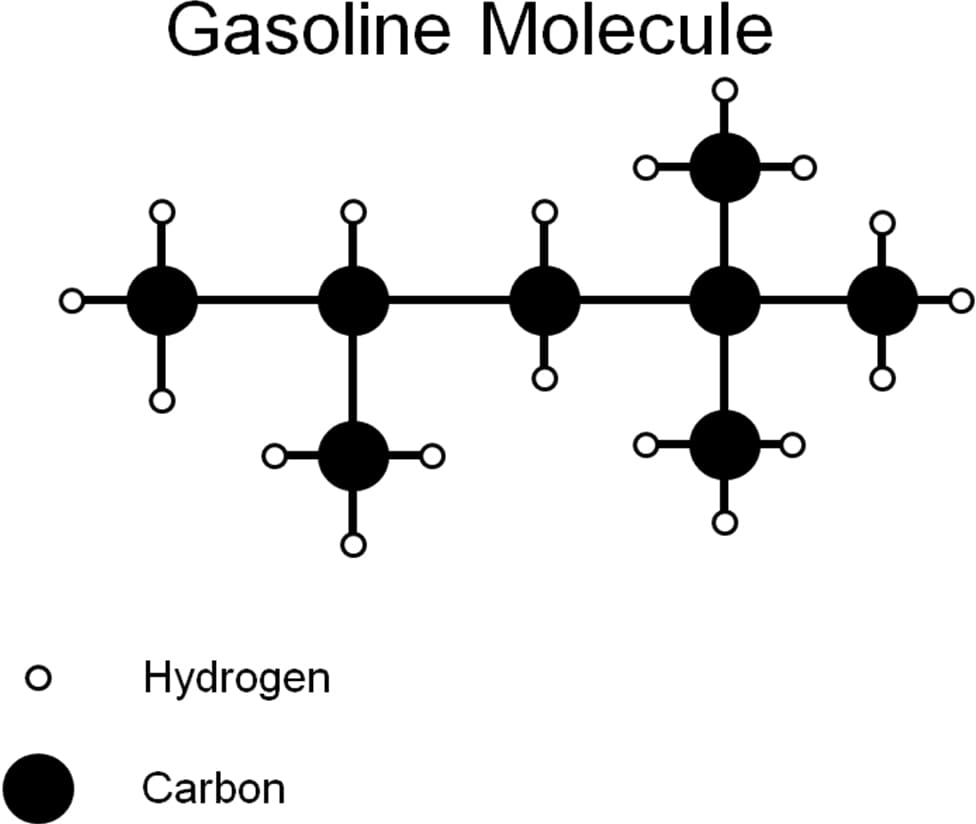
Fundamental Ideas:
1) Write a reaction for the complete combustion of gasoline to carbon dioxide and water.
2) Balance the equation based on the Costello Law of Thermodynamics
3) Explain in terms of the balanced equation how much carbon dioxide is produced per molecule of gasoline.
4) Based on atomic masses, explain the mass of carbon dioxide produced per mole of gasoline.
5) If you combusted 1000 g of gasoline completely, what is the mass of the carbon dioxide that would be produced? What is the mass of water vapor produced?
6) If gasoline weighs 6.073 lbs/gallon, and you burn 15 gallons of gasoline, how much carbon dioxide have you added to the atmosphere, assuming 100% combustion?

Comments: