Appendix 3
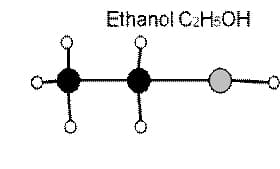
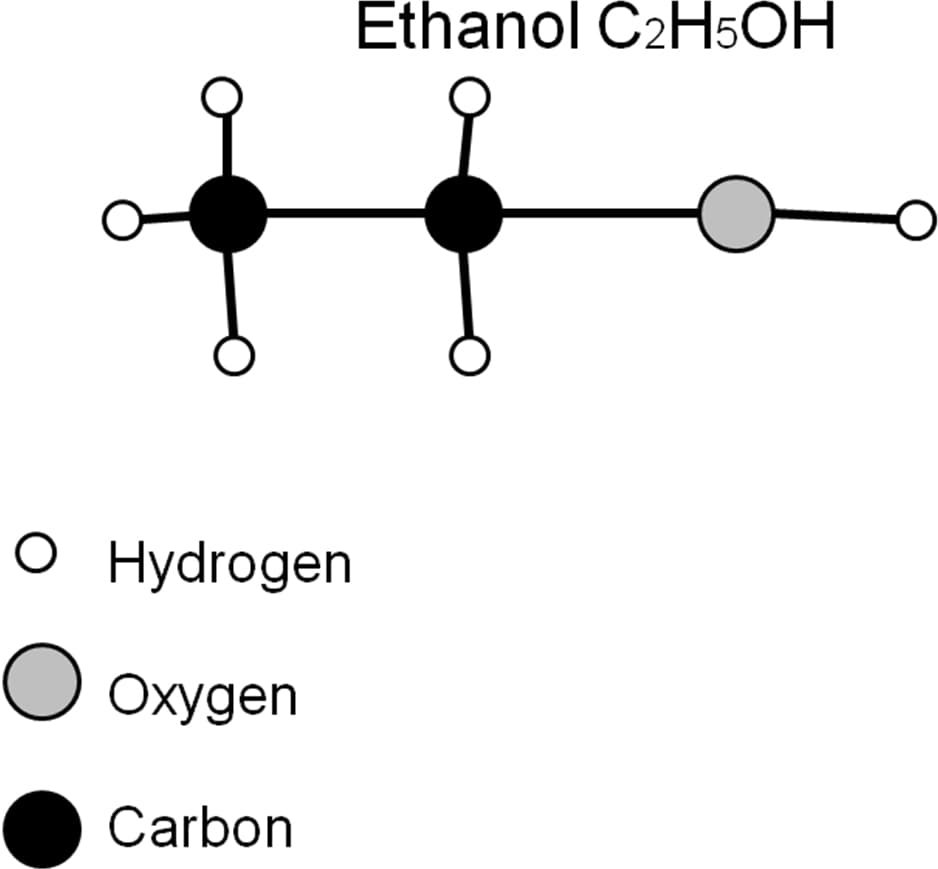
Average Bond Energies (kJ/mole)
C-C 348
C-H 413
C-O 358
O-H 463
Fundamental Ideas:
1) Using the above table of bond energies, calculate the total energy within one mole of Ethanol and one mole of Butanol.
2) Describe the relative amounts of energy as a ratio.
3) Compare the calculated energy content of Ethanol and Butanol to that of Gasoline.
4) Explain why one of the biofuels would be a better replacement for gasoline, based on energy content per mole.
Be the first to comment on this unit!

Comments: