Background
Immunity
Most organisms exhibit some version of an immune system that protects against harmful infections. Even unicellular organisms have a wide array of defenses against pathogens such as bacteria and viruses. In fact, many of these same defenses have been conserved or utilized in more recently evolved multicellular organisms 3. Most vertebrates, including humans, have two classifications of immune responses to protect against harmful invaders and internal sources of damage, such as cancer. These two types of responses are innate (nonspecific) immunity, and adaptive (specific) immunity.
Innate/Nonspecific Immunity
Innate immunity includes all factors that try to keep out invaders and which attack pathogens or harmful objects regardless of their identity. In humans, this type of immunity is the body's immediate defense against harmful substances, including its own cells whose mutations may cause harm to the organism as a whole. The body's preliminary innate defenses include the skin, mucosal membranes, hairs, and cilia. Even bacteria found in or on the body can reduce the potential of infection by other, harmful bacteria by outcompeting these bacteria for nutrients and binding sites. These mechanisms work to keep pathogens from ever entering the body or by trapping and "sweeping" them out before they can enter internal structures. Once a pathogen enters the body, or if harmful mutations form, the body has a variety of secondary defenses and mechanisms of protection. Many general feelings of malaise and sickness that people experience are results of the body's immune response. The achy feeling is actually the body's blood vessels expanding to increase the migration of white blood cells to the site of infection. Elevated body temperature, or fever, may create inhospitable environments for these pathogens and/or may improve the immune system's efficiency—the purpose is debated. 4 A variety of white blood cells, can work to phagocytize, or engulf, pathogens and degrade them. The most commonly referenced phagocyte is a macrophage. These white blood cells can also activate other white blood cells to help fight infection and can work as antigen presenting cells, which ties to adaptive/specific immunity, described below. Other white blood cells, known as natural killer cells, look for basic markers on cells, called Major Histocompatibility Complexes, or MHCs, that identify cells in the body as "self." Cells that do not have the form of MHC's found on that individual's cells are recognized as invaders or damaged and are stimulated to undergo programmed cell death; apoptosis. Since none of these interactions are specific to the type of pathogen infecting the body, they are all part of the nonspecific, or innate immune response. Innate responses, as a result, do not change as a result of a pathogen infecting the body more than once 5.
Adaptive (Acquired)/Specific Immunity
Soon after infection by a pathogen, an adaptive response begins to occur. Cells involved in adaptive immunity do more than recognize invaders as "non-self," they recognize, and respond to specific pathogens. This type of immunity is especially beneficial in its ability to "remember" specific pathogens and build a quicker response after a secondary infection. In fact, it is this natural benefit of adaptive immunity that has been exploited by scientists in the development of vaccines, which allow people to form immunity against pathogens before being infected. Vaccines will be discussed in more detail below. The cells involved in adaptive immunity are divided into two classes: humoral and cell-activated.
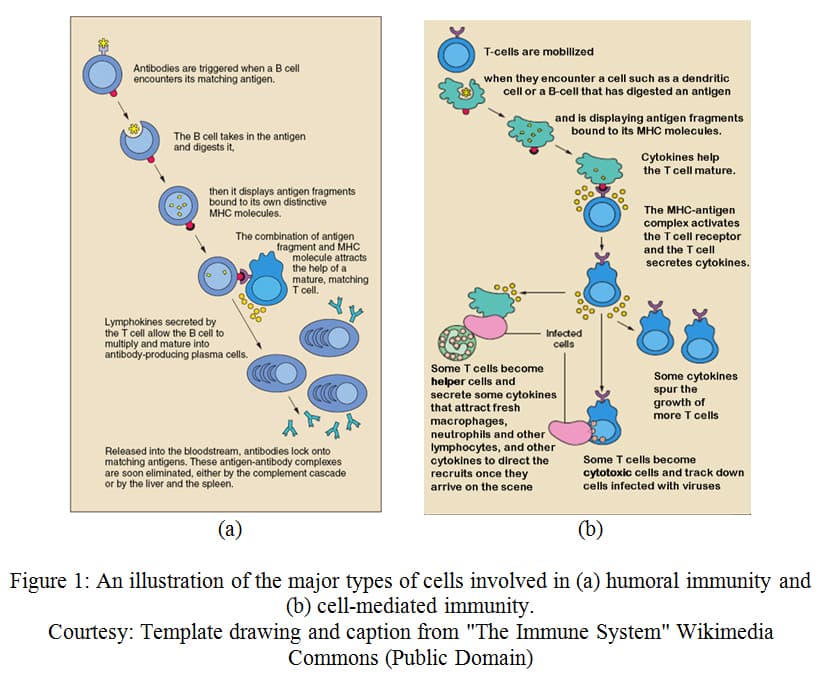
Humoral
Humoral immunity involves a specific type of lymphocytic white blood cell, called a B cell. B cells contain proteins on their outer membrane, called antigen receptors that recognize a specific antigen (invading pathogen, or mutant cell). In B cells, antigen receptors can recognize a variety of types of molecules from invading or mutated cells including surface proteins, polysaccharides, or even lipids. The antigens recognized can either be presented by an antigen-presenting cell, discussed later, or by a free-floating pathogen. When an antigen from one of these cells binds to the antigen receptor of a B cell, it, in effect, activates the cell and causes it to divide. Signals from T cells, another type of lymphocyte, cause these B cells to differentiate into either plasma B cells or memory B cells. 6 Plasma B cells are able to release antibodies, which, like the antigen receptors, recognize these specific antigens. Antibodies bind to the antigens and can neutralize the pathogen, mark them to be phagocytized by a macrophage, or signal to Natural Killer cells to stimulate apoptosis in this cell. 7 Most of these plasma cells undergo apoptosis once the cause of infection has passed. Memory B cells, however, remain in the body. Since these B cells are already developed against a specific antigen, upon secondary infection by the same pathogen, the body will be able to build an immune response much faster. Essentially, the body is remembering the pathogen so that it can remove the problem before the individual feels the effects of infection. It is important to remember that this immune memory is dependent upon the specificity of the antigen. Therefore, the strain of the pathogen must be similar enough to be recognized by the antigen receptors in order for a secondary immune response to be activated.
Cell-mediated response
The other type of adaptive immunity, known as cell-mediated response, involves another type of lymphocyte, called a T cell. Like B cells, T cells have antigen receptors. These receptors, however, are only able to recognize antigens that have been presented by an antigen-presenting cell, such as a macrophage. For example, in nonspecific immunity, a macrophage may phagocytize a bacterium. This macrophage will use enzymes to degrade the bacterium and will present parts of the degraded antigens on Major Histocompatibility Complexes (MHCs). There are two main types of T cells: Helper T cells (T H cells) and cytotoxic T cells (CTL cells). When the antigen receptor of a T H cell binds to the antigen presented by the MHC of a phagocytic cell, these T H cells are activated and stimulated to proliferate (expand in number). T H cells aid in the immune response by secreting chemical signals that activate other T cells, B cells, and other cells that play a role in the immune response. The other major types of T cells, CTL cells, are also activated when their antigen receptors bind to an antigen presented by an MHC. The type of MHC required for this process, however, is only found on certain types of cells. This binding will activate the CTL cells and cause them to multiply. The activated CTL cells may, for example, recognize and bind to an antigen presented by an MHC on a cell that has been infected by a virus. The CTL cell is able to perforate the infected cell and cause it to undergo apoptosis. Whereas helper T cells activate other types of cells, cytotoxic T cells are directly responsible for destroying cells that are infected with pathogens. In both cases, most T cells undergo apoptosis once the infection has cleared. Some, like with B cells, remain in the body as memory cells to generate a more efficient response in the instance of infection by the same pathogen at a later time. 8
Vaccines
In today's society, it is common to manipulate the immune system to keep people from ever getting sick by a disease. This is done through the use of vaccines. By exposing the body to an antigen, usually one that has been modified so that it no longer causes disease, the body can develop an immune response. If the body is then re-exposed to the actual pathogen, their body will already have memory cells for that antigen and will be able to quickly build an immune response "before it can inflict damage that results in symptomatic illness or death." 9 The first attempted control of a disease with vaccine, and the only disease ever eradicated through the use of vaccine, is smallpox. This vaccine is usually credited to Edward Jenner, though more recently he has been accredited with the research and publication of results that led to widespread vaccination.
In actuality, it is believed that variolation, a form of inoculation in which fluid from the smallpox-virus pustule of an infected individual was put beneath the skin of an uninfected individual, was practiced in Africa, India, and China in ancient times. The small exposure often reduced the likelihood of infection by smallpox virus from the environment. 10 It is no wonder that humans had tried to control the disease as smallpox had been wreaking havoc on the human species since sometime around 10,000BC. 11 In addition to causing the iconic pustules or "pox," infection by this virus led to disfigurement, blindness and, commonly, death. Smallpox has single-handedly affected the history of humans. In the late 1800s, the mortality rate from smallpox in Berlin was 98%. In 18 th century Europe, roughly 400,000 people died of smallpox per year. Of the survivors, 1/3 went blind. Smallpox nearly wiped out native populations in the New World as a result of introduction by European explorers carrying the virus. During the Middle Ages, smallpox decimated populations and greatly impacted civilizations. It may be linked to the fall of the Roman Empire and has even been discovered on mummified remains of Egyptian pharaohs. 12 Those that were lucky enough to survive a smallpox infection were immune for life. It was this observation that led to the spread of variolation to Europe. Though heavily influenced by social issues, over time variolation became a common practice in Europe and, eventually, in the New World. Though 2-3% of people died as a result of variolation, the mortality rate was much lower than the 14% of natural smallpox virus infection. 13
After taking much interest in the subject, and having been variolated himself, in the late 1700s Edward Jenner observed that milkmaids who had been infected with cowpox, a nonfatal disease, also seemed to have immunity to smallpox. Having already been extensively trained as a physician, surgeon, and researcher, he conducted an experiment in 1796. In this experiment, he infected an 8-year-old child with cowpox virus and, two months later, infected him with the smallpox virus. The boy did not get sick. Jenner published his findings and conducted follow up surveys. Eventually, the practice of using this vaccination (named after cowpox, "vaccinia") became common practice and, after the World Health Organization launched a global vaccine campaign, smallpox was eradicated in 1977. 14 With more information about the mechanism behind why this vaccine works, we have since been able to create vaccines for numerous pathogens.
Viruses
Many of the pathogens that commonly cause disease in humans, including smallpox, are viruses. Though it has been a cause for debate, viruses are generally considered nonliving due to the fact that they do not metabolize or respond to stimuli. These particles are often incredibly small, roughly 10 to 400 nm in size 15; viruses are not made of cells and require the replication machinery of host cells to reproduce. Viruses are, however, able to evolve and replicate. In fact, most of the genetic diversity of life is found within the genomes of viruses. 16 Life cycles differ by virus, which is discussed in more detail below. These structures of genetic material and protein have a massive impact on the environment. Infecting plants, animals, and in the case of bacteriophages, bacteria, these viruses impact population dynamics, community stability, and even abiotic factors such as global climate and oxygen production. In fact, it has been calculated that "10 percent of all the photosynthesis on Earth is carried out with virus genes." 17
Structure and Life Cycle
The structures of all viruses are the same in two ways: they contain an outer capsid, which is a structure made up of proteins, and they contain viral genetic material. Some viruses have envelopes inside their capsids while others do not, and the type of genetic material differs amongst viruses. 18 This size of viruses varies greatly, though the overwhelming majority of viruses are significantly smaller than bacteria. To provide perspective, about 1,000 viruses could be lined up along a grain of salt, in comparison to about 100 bacteria or ten skin cells. 19 The size of a virus does not correlate to the size of the host that it infects. In fact, one of the largest known viruses infects single-celled amoeba. 20 Viruses are grouped by the type of genetic material they contain (DNA or RNA, single or double stranded) and how the viral genome is replicated. 21
Viruses may undergo one of two life cycles: lytic or lysogenic. A virus begins infection when a part of its capsid attaches to a receptor on the outer membrane of a host cell. Due to the specificity of binding, each virus is only able to enter specific host cells. After attachment, the genetic material of the virus enters the cell and uses the host cell's replication machinery to make additional copies of its genetic material and the proteins required to form new copies of viral structures. If the virus is in a lytic cycle, this happens immediately and the virus erupts from the cell, causing lysis, or bursting of the host. Certain bacteriophages may also have a lysogenic life cycle, in which the viral DNA is inserted into the host DNA, remains dormant, and can be passed on through future generations. The period of dormancy is called latency and can vary in length. Eventually, reactivation can occur in which the viral DNA is copied, transcribed, translated, and new viral particles are assembled. This will result in the release of these viruses. 22 Animal viruses may also experience periods of latency in which viral DNA is inserted into the host genome and various lengths of dormancy occur. The exact mechanism of viral replication is particular to specific viruses and is linked to their type of genetic material. Retroviruses are of particular note because they use an enzyme called reverse transcriptase to turn their RNA into DNA. This DNA (referred to as cDNA) is then inserted into the host genome, which can then be used to make more viral RNA and proteins. It is also possible for the cDNA to be transcribed and translated without inserting into the host genome. Viral replication can occur so quickly that, upon entry of a cell, thousands of viruses can be synthesized within a day. 23 With latent infections, viral DNA can also remain in host genomes for prolonged periods, even so far as to be inherited by offspring. If mutations occur, these genes can remain in the genome permanently without the possibility of jumping back out of the host genome. In fact, approximately 8%, close to 100,000 fragments, of our own, human DNA is not human at all—but believed to be of viral origin. 24
Evolution of Viruses and their Impact on Human Evolution
Based on the life cycle and genetic diversity of viruses, it should be of no surprise that viruses are known for their rapid evolution. This can be attributed to recombination, high mutation rates, and short life cycles. DNA viruses, in general, have the lowest mutation rates among viruses, though single stranded DNA viruses have been found to have mutation rates only slightly lower than some RNA viruses. 25 The mutation rate in double stranded viral DNA is similar to the mutation rate in host DNA, but, as one study suggests, may result in more mutations overall due to the number of times this viral DNA is copied during a cellular infection. This study estimated the mutation rates to be 10 -8-10 -6 substitutions per nucleotide per cell infection. 26 RNA viruses, by comparison, lack molecular proofreading. The same study estimated that RNA viruses have mutation rates in the range of 10 -6-10 -4 substitutions per nucleotide per cell infection. Retroviruses have even higher mutation rates because reverse transcriptase "operates close to the error catastrophe threshold, the level of critical-copying fidelity below which information can no longer be maintained." 27 The mutation rates of all RNA viruses tend to be much lower if they insert their genetic material into that of the host cell. 28
In addition to mutations, viral genomes are able to recombine. In RNA viruses, for example, the RNA polymerase used to create more copies of viral RNA can switch the strands that it uses as a template. 29 Additionally, if a host cell is infected with more than one type of virus, the genomes of these viruses are able to recombine, resulting in a type of horizontal gene transfer between viruses. One study found that recombination rates were about 4% when a host cell was infected with two retroviruses. 30 High mutation rates, high recombination rates, and the speed at which viruses are replicated, all lead to the potential for rapid rates of evolution. These changes to viral genetic material can be deleterious, neutral, or positive. By increasing virulence or the ability for a virus to increase the survival time of its progeny, these viral mutations can be selected for, resulting in an overall improved fitness change of the viral population. These observations have also been used to explain why RNA viruses are less species specific and can more easily jump from one species to another, as is the case in zoonoses (animal acquired infections) and most emerging viruses. 31
The rapid evolution of viruses has also had a major impact on the evolution of other organisms. The phenomenon of host organisms' need to keep up with ever-changing viruses has been described as a "host-pathogen arms race." 32 It is hypothesized that humans and their ancestors have long been infected by DNA viruses, though these viruses likely had less of an impact on their evolution. This may also have been true of RNA viruses that have latent life cycles and, thus, are more stable. RNA viruses with high rates of evolution, on the other hand, more likely had an impact on human evolution. These viruses may have become more prominent when humans began domesticating animals and attracting disease-carrying rodents to their more permanent homes, allowing the viruses to more easily jump into humans. It is possible that some species of hominids were more susceptible to certain types of viruses, acting as "agents of selection." 33 The pressure of viruses may have encouraged genetic diversity in the human genome, especially when it comes to MHCs. It has even been suggested that in an earlier common ancestor, the "need for genetic diversity…contributed to the evolution of sexual reproduction." 34 The selective power of viruses on host genomes suggests the possibility of a type of coevolution between parasite and host. Retroviruses and DNA viruses that are able to insert their genomes into host DNA likely had a more direct role in changing the human genome, as can be seen with current human genes that have been identified as having originated as viral genes. The ability of humans and other placental mammals to develop a placenta is linked to an ancient virus-derived gene similar to that found in human endogenous retroviruses. 35 With the new evidence that viruses may have had a large impact on human evolution and their hominid relatives, it is clear that there is still much to learn about viruses.
HIV
HIV Infection
One virus that is of particular human disease interest is HIV. HIV, or human immunodeficiency virus, is the virus that causes Acquired Immunodeficiency Syndrome, AIDS. HIV continues to pose a major threat to humans. Worldwide, according to the CDC, about 35.3 million people are living with HIV infection, with 2.3 million new cases occurring each year. In 2012, an estimated 1.6 million individuals died as a result of AIDS. In the United States, approximately 1.1 million people are infected with HIV, 16% of whom are unaware of their status. 36 In Washington D.C. (as of 2009), 3% of the population is infected with HIV. This rate is twice as high for African American males. 37 In 2011, Chicago reported an HIV prevalence rate that was 3 times greater than the national average, with HIV-infection and AIDS-diagnosis rates that were twice the national averages. 38
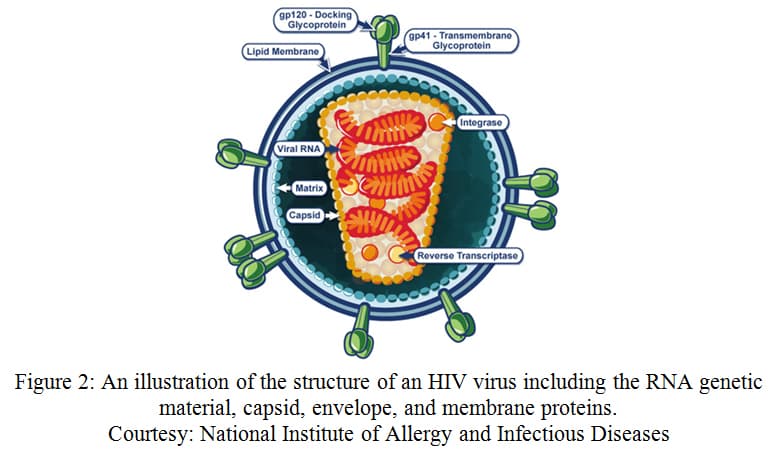
While there is no cure for AIDS, the impact of HIV on the human population has promoted extensive research of HIV's structure, life cycle, and infection mechanisms. HIV is a single stranded RNA (ssRNA) virus that has two copies of its viral genome. These viral RNA molecules are non-covalently linked at their 5' end. 39 The virus itself is composed of an envelope made up of a lipid bilayer with viral proteins. Beneath this envelope is the capsid, within which is the viral RNA and reverse transcriptase enzyme. When HIV encounters a CD4+ T cell (a type of helper T cell), the virus attaches to CD4 receptors and co-receptors (usually CCR5 or CXCR4). This attachment allows the viral capsid to enter the T cell. Reverse transcriptase uses the viral RNA to create a DNA strand within this capsid. This viral DNA is inserted into the host genome and, eventually, used to make more copies of the viral RNA and viral proteins. This allows the virus to escape the cell and infect more CD4+ T cells. 40 AIDS is established once the number of surviving CD4+ T cells falls below a certain level, compromising the immune system of the individual.
There are two main types of HIV: HIV-1 and the less virulent form, HIV-2. It has been hypothesized that HIV-2 emerged as a result of a bite to a human from a mangabey monkey, which carries a similar lentivirus. 41 HIV-1 likely jumped into the human population from chimpanzees when they were killed for meat by hunters. This did not pose a problem to the global population until these hunters began entering cities and the virus spread between humans. HIV-1 is estimated to have been in the human population since roughly 1933, though it wasn't recognized until 1983. 42 HIV-1 is further split into groups: M (main), O (other), and N (non-M; non-O), each of which is predicted to have independent origins of transmission. 43 While Types O and N are only found in Africa, Group M is found everywhere and has been split into subtypes A-D, F-H, J, and K. Sub-Saharan Africa has the greatest diversity of HIV-1 representing subtypes A, C, D, F, G, H, J, K. 50% of the HIV infections worldwide are subtype C, though Subtype B is the most highly represented in the Americas, Western Europe, and Oceana. 44
Current HIV Treatment
Current treatment for HIV includes the use of a cocktail of drugs referred to as Highly Active Anti-Retroviral Therapy, or HAART. These drugs work by inhibiting reverse transcriptase, keeping viruses from replicating, and by stopping HIV viruses from entering CD4+ T cells in the first place. In recent years, these drugs have been fairly successful in allowing people to live mostly normal lives. Still, these treatments are extremely expensive, and, as such, "has meant that most people with HIV—living in the poorest countries—cannot afford a treatment that might give them extra years or even decades of life." 45 Still, these drugs have negative side effects that can be harmful to humans after years of treatment and encourage the proliferation of viruses that are unaffected by these drugs. 46 This becomes especially concerning when it is recognized that 26% of new HIV infections in the United States occur in people ages 13-24. 47 Dr. Anthony Fauci, the head of the National Institute of Allergy and Infectious Disease, has continued to conclude that, "the development of an HIV vaccine must remain at the top of the global health research agenda." 48
The Struggle to Develop a Vaccine
Though HIV vaccines have been researched for over 20 years, no successful vaccine has been developed and released to the public. Though some vaccines have made it to trial stages, none have been approved for public use. In 2008, a vaccine developed by Merck went through human trials. These trials were shut down after it was found that the vaccine was actually making people more susceptible to HIV infection rather than less. 49 There are a variety of reasons that scientists have had a hard time developing a vaccine against HIV. Most classical vaccines mimic the response some individuals are able to naturally mount against the infection of that pathogen, as is exemplified with the smallpox vaccine. This concept is called "proof of concept." 50 While some individuals are able to survive for longer periods of time with an HIV infection before succumbing to AIDS, no one has ever been cured of an HIV infection. Scientists are faced with the challenge of creating a vaccine that "produces a protective immune response that is superior to that elicited by natural infection." 51 The elusiveness of HIV can be attributed to its ability to establish latency, its high mutation rate, its high likelihood of recombination, its ability to evade immune system cells, and its direct attack on the immune system itself.
As previously stated, HIV is a retrovirus and as such, has a high mutation rate. One study calculated HIV's mutation rate to be 4x10 -5 mutations per target base pairs per replication cycle in T cells. 52 A different study found that homologous recombination is also frequent in HIV-1 viruses, occurring 2.4x10 -4 times per base pair per replication cycle. This can take place when the reverse transcriptase switches the template strands, known as "copy choice." 53 Increase in HIV diversity through recombination would be particularly high in cases that a host cell is infected by more than one strain of HIV.
These rapid changes in HIV's genome contribute to its ability to evolve quickly. In a study conducted by Song, et al., the change in viral genotypes was analyzed in vivo. Within 2 weeks, the original strain of HIV made up less than 10% of the HIV population. Of the other genotypes, 2 main mutations were identified. One mutation allowed HIV viruses to escape cytotoxic T lymphocyte (CTL) detection. Mutations such as these are referred to as CTL escape mutations. These mutations have previously been found to have negative affects on viral replication. A different mutation was categorized as a reversion mutation. The majority of viruses analyzed had both of these mutations within 14 days. 592 days after initial screening, 100% of the cells had both mutations. 54 Additionally, these viruses were shown to not suffer reduced replicative fitness. 55 This information suggests that the selective pressure of CTL cells reduced other HIV strains, allowing the mutated HIV to proliferate and increase their capacity to evade the immune system. Similarly, in the case that scientists were able to develop a vaccine, the high mutation and recombination rates of HIV would likely make these vaccines ineffective. The evolution of HIV has also allowed it to evade the immune system by changing the carbohydrates and proteins the immune system would use to identify HIV. 56 At the same time, HIV quickly establishes latency in host cells, "likely within days." 57 This dormant period allows the viruses to hide from the immune system, remaining undetectable until they reactivate and spread throughout the body. It is as if, as the body wages war against the viruses it can see, HIV is building an unseen army, ready to be deployed at any time.
Some positive discoveries have been based around conserved sequences of one of HIV's surface proteins, gp120. This protein allows HIV to enter host cells and, therefore, cannot change much or HIV will lack its invasion abilities. Scientists have begun to find antibodies that are capable of neutralizing HIV and recognize this conserved sequence. One such antibody, announced in 2010 called VRC01, was found to neutralize 90% of common HIV-1 strains. 58 Still, administering antibodies is currently a method of treatment rather than prevention. It will be a challenge for scientists to determine how, if these are used to create a vaccine, these antibodies could be induced in the majority of people. 59 Other scientists have begun to investigate the population of people in Europe who are naturally immune to HIV infection due to a mutation in their CCR5 co-receptor. In one study, an individual underwent successful stem cell transplant with blood stem cells that were homozygous for the mutated genes. The results reported that after 20 months, HIV-1 could not be detected in the individual, indicating control of the HIV infection. Still, scientists are concerned with mutated strains of HIV-1 known to bind to a different co-receptor. Individuals with the CCR5 mutation would not be immune to these strains of HIV. 60 Additionally, the challenging and invasive stem cell transplants present as a treatment, rather than preventative, option. As scientists continue to explore preventative options, Dr. Fauci suggests that, perhaps, scientists need to think unconventionally and investigate manipulating the innate immune response in a way to "better protect the body at the most frequent points of entry of the virus" such as the mucosal membranes. 61 As the search for a preventative vaccine continues, it is clear that rapid evolution of the virus continues to elude both the human body and scientists.

Comments: