BACKGROUND CONTENT
Life Cycle Assessment
The Life Cycle Assessment (LCA) “offers a method for quantitatively compiling and evaluating the inputs, outputs and potential environmental impacts of a product system throughout its life cycle” .6
The LCA processes date back to the early 1960’s and have continued to evolve in the ensuing decades with regular updates issued by the ISO (International Standards Organization). The following description of the current LCA standards and procedures is based on the most recent update (ISO 14040) issued in 2006. The steps in the LCA process are depicted in Figure 1.
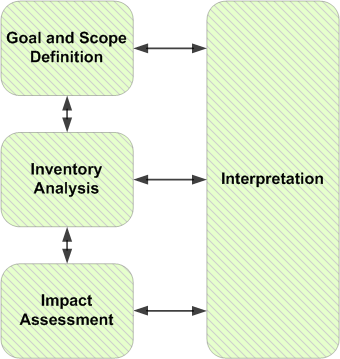
Figure 1 Life Cycle Assessment Framework:
Source:https://commons.wikimedia.org/wiki/File:PhasesOfLifeCycleAnalysis.png
Goal and Scope Definition
The LCA follows a series of steps as depicted in figure 1. The process typically begins with a definition of goals and a clear definition of the product (or product system) and the scope of the investigation. The purpose of this step is to identify all aspects of the product’s life cycle, as well as the emissions and all related environmental impacts. It is important to note that although this is seen as the first step, the process (as shown by the arrows between the steps) is iterative, as evaluators often need to review and adjust their findings at any stage of the analysis.
An essential component of the scope is the definition of the function of the product (or product system) that will be the focus of the analysis. Clearly identifying the product’s function is important given that LCA’s are often used to compare the environmental impacts of various products. Such comparisons are only valid when similar specific functions are analyzed 7
For example, to compare the sustainability of differing packing materials, an LCA compared
popcorn (PC) vs. polystyrene (PS).8 The LCA found that the manufacture of popcorn requires less energy and produces less CO2 emissions than the polystyrene packets (which are made from fossil fuels and are not biodegradable). However, the density of popcorn is 4.6 times that of polystyrene, meaning that it would require a smaller amount of polystyrene (given its smaller density) to fill the volume of a packing box. If packaging is the function the LCA is assessing,
then polystyrene is the more sustainable option as a smaller quantity will be needed as packaging agent .9 Clearly identifying the function is a critical first as the focus of LCA is to relate environmental impacts to the function of a product over its whole life cycle. Given that LCA’s are used to quantitatively compare product systems across various scenarios, the functional unit provides a numerical context that can be used to compare product function.
A functional unit is a quantified description of the performance of the product systems, for use as a reference unit. Example: Lighting 10 square meters with 3000 lux for 50000 hours with daylight spectrum at 5600 K.10
Rare Earth Elements do not have a specific end use as they are primarily used as intermediate components of various products. As such they have no specific function or functional unit rather, they are assigned a reference unit. The reference unit used in their LCA is defined as 1kg of separated REEs comprising the set of elements in the lanthanide series along with yttrium and scandium.11 The product function helps determine the system and system boundaries. The system is the set of processes that come together to perform the system’s function. The processes in the refining of REEs (mining and beneficiation, chemical treatment, separation, reduction, refining, and purification of ores .12 The inputs into the system are resources needed to complete a given process (i.e. chemicals, energy, water, land area). Outputs are the substances that exit the system into the environment: (emissions to the air, water, and, soil), chemical byproducts etc. It is imperative that all economic processes ( i.e. energy, raw materials, product manufacturing, infrastructure production) that input the system, as well as all the outputs ( emissions, waste processing) are identified.13
Inventory Analysis
LCA phase involves “the compilation and quantification of inputs and outputs for a product throughout its life cycle”.14 It is during this phase that all inputs and outputs of the various processes as well as any subprocesses in the system are linked together as a flow of interconnected unit processes. Once the size of each of the inputs is determined the scale of the inputs per unit of output can be determined.
Impact Assessment:
The Life Cycle Impact assessment is the “ phase of life cycle assessment aimed at impacts for product systems throughout the life cycle of the product.15 This last phase of the assessment uses data from the inventory analysis to evaluate the potential environmental impacts of the product. Evaluators use established methodologies (Eco-indicator, ReCiPe, CML) to classify the impacts into categories such as, ‘global warming, eutrophication, acidification, mineral depletion, etc.).16
Interpreting Results
The assessment ends with the creation of a summary statement that includes the conclusions of the evaluators and recommendation for the relevant stakeholders. It is important that the interpretation be consistent with the goals and scope determined in the first phase of the assessment.
Examples of LCAs
Life Cycle Assessments are used to evaluate products in a wide range of products, technologies, and industrial processes. Each follows the steps outlined in LCA framework. A list of representative LCAs is included in the Appendix. They will be used as part of student’s independent research.
The Chemistry of Rare Earth Elements
Rare earth elements are a group of 15 elements (beginning with #57 (Lanthanum) and ending at # 71 Lutetium) that are known as the Lanthanide series. Although Scandium # 21 and Yttrium # 39 are not members of the lanthanides, they are included in this group because they share similar physical and chemical properties and are usually found in mineral deposits with other REEs.
The earth elements are separated into three groups based on their chemical and physical properties: ( Light REEs ( Lanthanum #57 to Europium # 63); Heavy REEs (Gadolinium #64 to Lutetium #71, Sc# 21, and Yttrium # 39); and the Middle REE’s (Europium, Samarium, and Gadolinium).
Chemical and Physical Properties
Rare earth elements are usually together in metallic complexes as they share similar ionic radii and are mostly trivalent (3+ charge). Exceptions are Cerium (Ce4+ the most abundant REE) and Europium (Eu 2+). They also share a somewhat similar electronic configuration as each, beginning with La ( [Xe] 6s2 5d14f0 to Lu [Xe] 4f14 5d1 6s2), is adding an electron to an inner 4f orbital (Table 1). The addition of an electron to an inner electron shell shrinks the atom’s atomic and ionic radius across the period. This trend (knows as the “lanthanide contraction” is responsible for the lanthanides unique magnetic and optical properties.17 The contraction is especially important in the mining and processing of REE as it creates a fractionation effect which allows for the separation of REE complexes found in soils and rocks.
Table 1: Physical Properties of Rare Earth Elements
|
Element |
Symbol |
Atomic number |
Atomic weight |
Electron Configuration |
Crustal abundance (ppm) |
|
Light REEs |
|||||
|
|
|||||
|
Lanthanum |
La |
57 |
138.9 |
[Xe] 4f0 5d1 6s2 |
39 |
|
Cerium |
Ce |
58 |
140.12 |
[ Xe] 4f2 6s2 |
66.5 |
|
Praseodymium |
Pr |
59 |
140.91 |
[Xe] 4f36s2 |
9.2 |
|
Neodymium |
Nd |
60 |
144.24 |
[Xe] 4f4 6s2 |
41.5 |
|
Promethium |
Pm |
61 |
[Xe] 4f5 6s2 |
~ 0.0 |
|
|
Samarium |
Sm |
62 |
150.36 |
[Xe] 4f6 6s2 |
7.05 |
|
Europium |
Eu |
63 |
151.96 |
[Xe] 4f76s2 |
2.0 |
|
Gadolinium |
Gd |
64 |
157.25 |
[Xe] 4f7 5d1 6s2 |
6.2 |
|
Heavy REEs |
|||||
|
Yttrium |
Y |
39 |
88.91 |
[Kr] 4d1 5s2 |
33 |
|
Terbium |
Tb |
65 |
158.92 |
Xe] 4f9 6s2 |
1.2 |
|
Dysprosium |
Dy |
66 |
162.50 |
Xe] 4f10 6s2 |
5.2 |
|
Holmium |
Ho |
67 |
164.93 |
Xe] 4f11 6s2 |
1.3 |
|
Erbium |
Er |
68 |
167.26 |
Xe] 4f12 6s2 |
3.5 |
|
Thulium |
Tm |
69 |
168.93 |
Xe] 4f13 6s2 |
0.52 |
|
Ytterbium |
Yb |
70 |
173.04 |
Xe] 4f14 6s2 |
3.2 |
|
Lutetium |
Lu |
71 |
174.97 |
Xe] 4f14 5d1 6s2 |
0.8 |
Applications and Uses
It is difficult to describe all the ways that REEs are used in our technological society as they have become indispensable to a wide range of industrial and technological applications. They are increasingly important in “green technologies” as they are essential components in permanent magnets used in wind turbines, high capacity NiMH batteries in electric vehicles and as phosphors in compact fluorescent lamps. They are extensively used by the glass industry to color glass and polish the surfaces of flat screen displays. Lanthanum and lutetium are used to alter the refractive index of optical glass. Other REEs are used to give glass filtering and glare reducing qualities. The petroleum industry uses lanthanum-based catalysts in refining processes while cerium and trace amounts of other REEs are used in the catalytic converters in most vehicles. They are as noted earlier essential components of permanent magnets, nickel-metal hydride batteries, and in all phosphors used in flat panel displays, light emitting diodes, medical devices, and lasers. These are but some of the ways that REEs are used in our world. A complete analysis of their many applications would require a very lengthy narrative. Table 2 describes some of their many other uses.
Table 2: Applications of Rare Earth Elements
|
REE |
Applications Products |
|
Scandium |
Aerospace materials, electronics, lasers, magnets, lighting, sporting goods |
|
Yttrium |
Ceramics, communication systems, lighting, frequency meters, fuels additive, jet engine turbines, televisions, microwave communications, satellites, vehicle oxygen sensors |
|
Lanthanum |
Catalyst in petroleum refining, television, energy storage, fuel cells, night vision instruments, rechargeable batteries |
|
Cerium |
Catalytic converters, Catalyst in petroleum refining, glass, diesel fuel additive, polishing agent, pollution-control systems |
|
Praseodymium |
Aircraft engine alloy, airport signal lenses, catalyst, ceramics, coloring pigment, electric vehicles, fiber optic cables, lighter flint, magnets, wind turbines, photographic filters, welder’s glasses |
|
Neodymium |
Anti-lock brakes, air bags, anti-glare glass, cell phones, computers, electric vehicles, lasers, MRI machines, magnets, wind turbines |
|
Promethium |
Beta source for thickness gages, lasers for submarines, nuclear powered battery |
|
Samarium |
Aircraft electrical systems, electronic counter measure equipment, electric vehicles, flight control surfaces, missile and radar systems, optical glass, permanent magnets, precision guided munitions, stealth technology, wind turbines |
|
Europium |
CFL, lasers, televisions, tag complex for the medical field |
|
Gadolinium |
Computer data technology, magneto-optic recording technology, microwave applications, MRI machines, power plant radiation leaks detector |
|
Terbium |
CFL, electric vehicles, fuel cells, televisions, optic data recording, permanent magnets, wind turbines |
|
Dysprosium |
Electric vehicles, home electronics, lasers, permanent magnets, wind turbines |
|
Holmium |
Microwave equipment, color glass |
|
Erbium |
Color glass, fiber optic data transmission, lasers |
|
Thulium |
X-ray phosphors Improving |
|
Ytterbium |
Improving stainless steel properties, stress gages Catalysts, |
|
Lutetium |
Catalysts, positron emission tomography (PET) detectors |
Geology of REEs
In nature REEs are most commonly found in minerals formed from carbonatites (igneous carbonate rocks), in carbonates ( flurocarbonates, hydroxyl carbonates) and in silicates, oxides and phosphates. The most important REE bearing mineral is Bastnaesite (REE)(CO3)F as it is the primary mineral in the world’s largest REE mines: the Bayan Obo mine in China and the Mountain Pass Mine in California.18 Other important REE minerals are Monazite (REE, Th)(PO4), Xenotime (YPO4), and Ion adsorption clays: 2(Kaolin)3-RE3+ . The processing of these elements begins with their extraction from the earth.
Production of REE Follows Three Stages
The processing of REES begins with mining of RE containing iron ores. Mining practices range from placer mining, underground to open pit mining. The type of mine depends on the site and ore type. Both Bayan Obo and the Mountain Pass mine use open pit methods to extract REE ores. Following extraction, the RE minerals are separated from gangue (waste rock components) and produce minerals that contain approximately 50% rare earth ores (REO).
Beneficiation is used to improve the quality of the ore by grinding, sifting, gravity or magnetic filtration or froth flotation. This phase of the refining process carries the first of many environmental risks. The mechanical separation processes pose minimal risks as they require little heat and use no chemicals. Froth flotation used with bastnaesite ores, employs an array of acids (organic phosphoric acids, dicarboxylic acids) as collectors (compounds that make metals more hydrophobic) thus increasing their separability. Additional compounds (sodium silicates, sodium hexafluoro-silicate, and sodium carbonate are often used as depressants ( chemicals used to inhibit the flotation of gangue minerals). The chemical residues from these and other refining processes collect in tailing ponds where they mix with wastewater, heavy metals, radioactive elements (thorium is naturally associated with REEs) that pose serious environmental risks for the ecosystems and people living near the mines 19
The concentrates formed during beneficiation are then treated with a series of acids and electrolytes that purify and increase their REO concentration to approximately 90%. Another process commonly used is high acid roasting that emits hydrogen fluoride, sulfur dioxide and trioxide and silicon tetrafluoride gases. Several scrubbers are used to capture and purify the exhaust gases.
In the final step, solvent extraction that exploits differences in basicity to separate the individual REO’s. This process is necessarily slow given the slight difference in basicity between REEs. Once separated, reduction using molten salt oxide, or fused salt electrolysis can be used to separate the individual REE’s from their ores.20 Figure 2 is a representation of the system processes for the refining of REEs from bastnaesite and monazite ores.
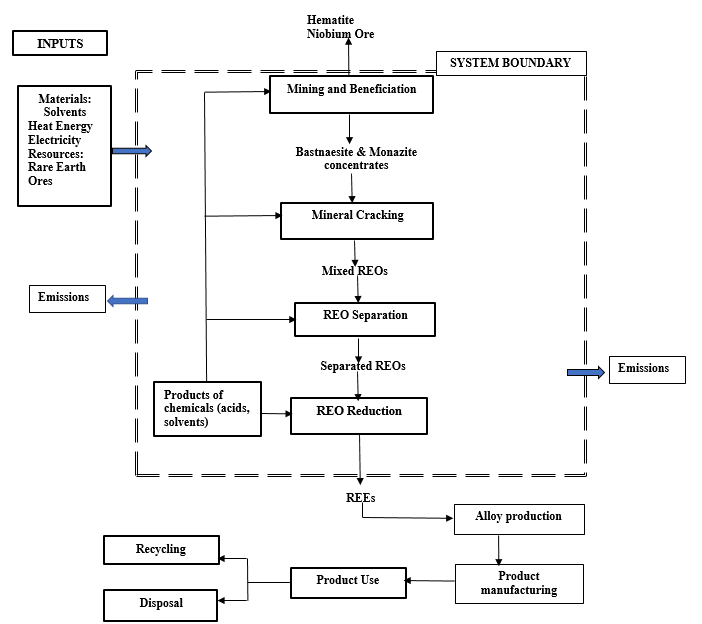
Figure 2: Rare Earth Element System Boundary with Inputs and Outputs for Production of Rare Earth Elements from Bastnaesite Ore. Source: 21
Environmental Concerns
Scientists say that under regulated rare earth projects can produce wastewater and tailing ponds that leak acids, heavy metals and radioactive elements into groundwater, and they point out that market pressures for cheap reliable rare earths may lead project managers to skimp on environmental protections 22
In 2002 the only mine in the US, the Mountain Pass was closed because of continued leaks of radioactive wastewater. In China, Malaysia, Australia, and other countries citizen groups are mounting campaigns against rare earth mining given the real ( and under investigated) threats these mines pose to human health and ecosystems.23 Exposure to radioactive soils and water remain an ongoing concern for those living near REE mines because rare earth elements are often complexed with radioactive elements like thorium. The many emissions created in the processing of these ores have been implicated in the increased incidence of cancers, diabetes and other health concerns in villages surrounding the Bayan Odo mines. The toxic mix of chemicals found in the tailing ponds has contaminated the soils and groundwater for miles around the mines.24
Table 3: Environmental Impacts per kg of metal from the Production of Rare Earth Elements at the Bayan Obo Mine: China: Data Source 25
|
REE |
Energy Consumption |
Water |
Environmental Impact |
|||
|
Elec. |
Heat Energy |
Human |
Ecosystem |
Resource Depletion |
||
|
Lanthanum |
91.8±17.8 |
127.6±27. |
43.5±11.0 |
7.38±1.32 |
3.48±0.88 |
44.3±10.6 |
|
Cerium |
154.7± 29.0 |
199.5±41.5 |
75.7±19.1 |
12.40±2.22 |
5.89±1.50 |
74.3±18.2 |
|
Praseodymium |
91.9±17.9 |
128.2±27.3 |
43.4±11.0 |
7.38±0.33 |
3.48±0.87 |
44.3±10.6 |
|
Neodymium |
173.1±33. 3 |
218.9±45.2 |
85.5±21.4 |
13.85±2.47 |
6.59±1.69 |
83.0±20.4 |
|
Gadolinium |
996.4±19 1.0 |
1166±235.0 |
522.3±131.7 |
79.80±14.19 |
38.12±9.89 |
481.6±120.2 |
|
Yttrium |
331.5±636 |
424.4±86.8 |
180.7±45.9 |
26.82±4.77 |
12.81±3.31 |
165.7±40.8 |
|
|
|
|
|
|
|
|
Environmental Impact Units: Source 26
DALY*10 - Disability Adjusted Life YearsDALY = YLL (Years of life lost) + YLD ( years of life disabled) * Q (Quality of life)
Q = 0 (optimum health) : Q = 1 (Life Lost).
Damage to Ecosystem: PDF*m2 * yr –Potentially Disappeared Fraction of species * area over which they disappear * number of years of damage.
Addressing Environmental Impacts: The need for recycling
The increasing demand ( and limited supply) for rare earths, has led many has led many countries to open new mines or reopen existing sites: ( the Mountain Pass mine closed in 2002 is due to reopen later this year 27) . This is unfortunate as the LCA analysis of REE has clearly shown the environmental damage caused by the mining and processing of these elements. While much attention is given to developing new resources, research has shown that less than 1% of these elements are recycled.28 Adding additional resources through recycling efforts would increase supply and likely lessen the need for new mines. Recycling of REE can occur during the mining, manufacturing and refining phases where resources can be recovered from waste residue and scrap or they can be found in from post-consumer “end of life” products through urban mining 29 and landfill mining.30
Recycling Methods
This review of recycling methods focuses on three applications that comprise approximately 80% of the rare earth market: permanent magnets (38%), nickel metal hydride batteries (13)% and lamp phosphors (32%). Table 4 lists the % use of REE in each of these applications. Recycling the end of life products in these applications yields
(See Table 2 for a detailed list rare earth element usage by %). The following protocols will serve as an introduction to recycling methods.
(Note: Students will be able to explore the recycling of REEs in other products as part of their engineering design activity. Additional information on recycling REEs used in other applications and their methods will be provided as the need arises).
Table 4: Rare Earth Usage by application in %: Source:(Curtis et al (2011) 31
|
Application |
La |
Ce |
Pr |
Nd |
Sm |
Eu |
Gd |
Tb |
Dy |
Y |
Other |
|
Magnets |
23.4 |
69.4 |
2 |
0.2 |
5 |
||||||
|
Battery Alloys |
50 |
33.4 |
3.3 |
10 |
3.3 |
||||||
|
Metallurgy |
26 |
52 |
5.5 |
16.5 |
|||||||
|
Auto Catalyst |
5 |
90 |
2 |
3 |
|||||||
|
FCC |
90 |
10 |
|||||||||
|
Polishing powders |
31.5 |
65 |
3.5 |
||||||||
|
Glass additives |
24 |
66 |
1 |
3 |
2 |
4 |
|||||
|
Phosphors |
8.5 |
11 |
4.9 |
1.8 |
4.6 |
69.2 |
|||||
|
Ceramics |
17 |
12 |
6 |
12 |
1 |
53 |
|||||
|
Others |
19 |
39 |
4 |
15 |
2 |
1 |

Comments: