Introduction
Look around you and try to identify anything that is made of or contains plastic. Notice your car, laptop, phone, clothes, food packaging, children’s toys, etc.; all of which contain some sort of plastic. Plastic is a necessary material for our modern-day living. However, would you be surprised to find out that plastic’s popularity only just began to rise in the late 1950s? As you can see in Figure 1 below, the production of plastic has grown exponentially since 1950. The favorable characteristics of this synthetic polymer are what makes its use so widespread. The terms “synthetic” and “polymer”, when used to describe plastic, can most simply be defined as many chemical units repeated and put together. Each type of polymer has its own unique characteristics, but most plastics share some similarities. Plastics are typically low density, inexpensive, durable, can be processed in many different ways depending on the function it will serve, and they can be used to make products not accessible in the natural world.1 The allure of this synthetic material is enticing, but there is a caveat. Plastics are not biodegradable, meaning they cannot breakdown completely and can thus accumulate all over our world.
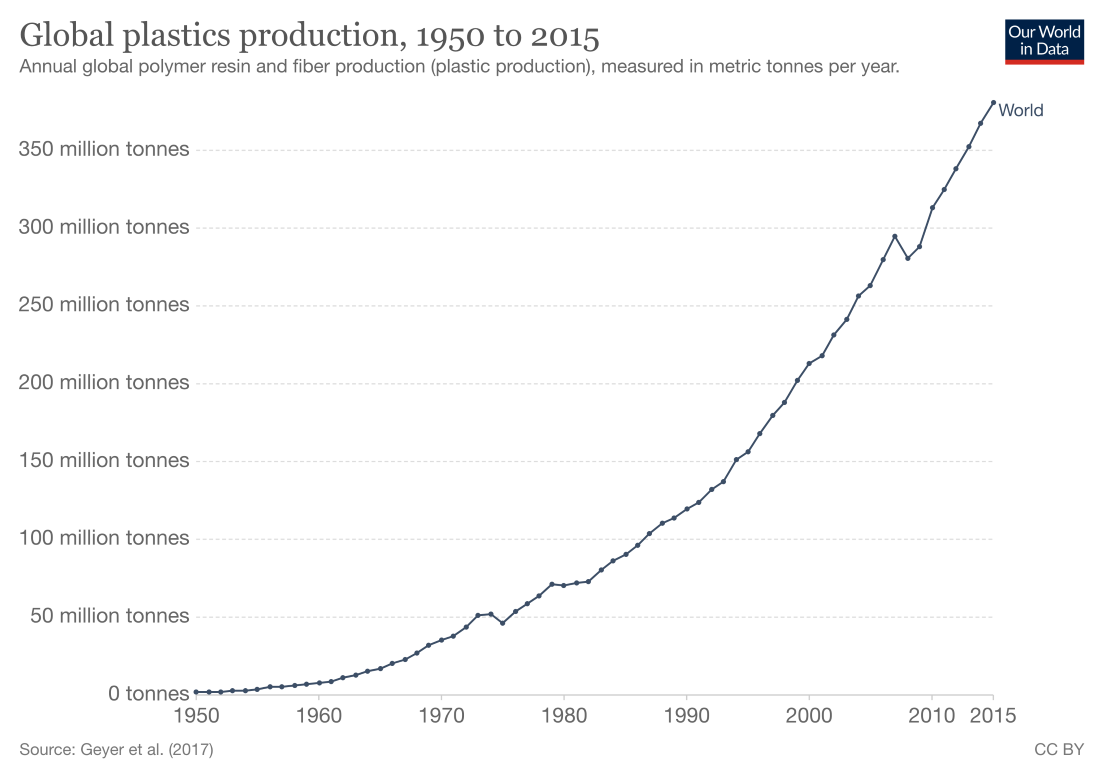
Figure 1. Graph depicitng plastic production in metric tons per year.2
The rise in plastic production grew from two metric tons in 1920 to 380 metric tons in 2015.3 In 2017, the EPA reported the average person generated four and half pounds of waste every day. As a nation, the U.S. generated approximately 268 million tons of waste in that year. Of those 268 million tons, plastic made up about 13% or 35 million tons. When we only consider plastic waste, 75% was placed into landfills or ended up in the natural environment, 16% was combusted with energy recovery, and only 8% was recycled.4 A visual representation of the plastics waste management is provided in Figure 2 below.
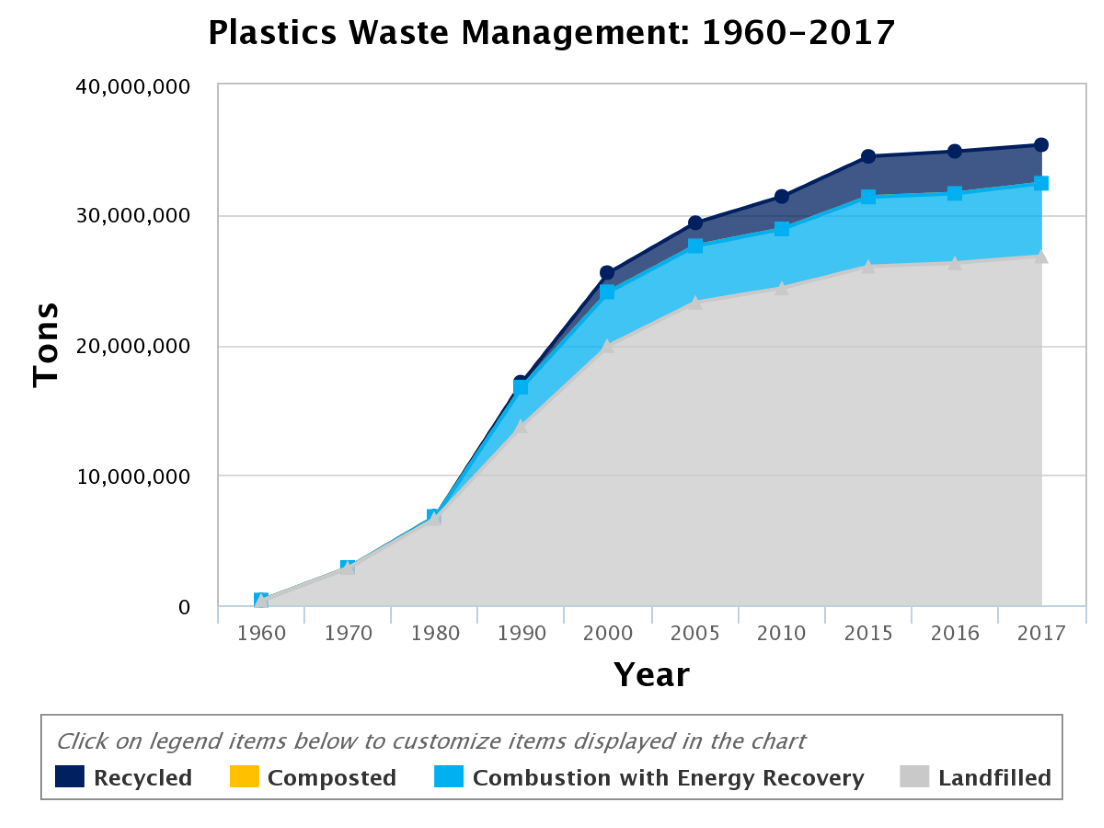
Figure 2. Graph depicting plastic waste management methods by tons.5
If the trends of plastic production and waste continue at the this rate, about 12,000 million metric tons of plastic waste will accumulate in landfills or the natural environment by 2050.6 The contamination of the natural environment with plastic waste should be of paramount concern. When plastic waste enters the environment, whether it be a landfill or as litter, UV exposure and mechanical degradation will break down larger plastic items into smaller microplastics, which range micrometers to millimeters in size.
“Microplastics are of special concern since their bioaccumulation potential increases with decreasing size. Microplastics may be ingested by various organisms ranging from plankton and fish to birds and even mammals, and accumulate throughout the acquatic food web. In addition, plastics contain a multitude of chemical additives and absorb organic contaminants from the surrounding media. Since these compounds can transfer to organisms upon ingestion, micorplastics act as vectors for other organic polluntants and are, therefore, a source of wildlife exposure to these chemicals.”7
While the numerous beneficial characteristics of plastics make them a necessity for today’s society, those same characteristics are now presenting many challenges to environmental health. Countries are responding to those challenges with new environmentally-friendly policies, initiatives, and engineering. Within this unit, there are three overarching goals to be achieved. First, it aims to address the basic chemistry of plastics exploring the basic chemical synthesis of polymers. Second, the life cycle of petroleum-based plastics is addressed, which will reveal the challenges of plastic waste. Third, students will consider the future of plastics utilizing their newly acquired knowledge of current pletroleum-based plastic production and disposal. They will address the need for more sustainable practices in synthesizing plastics by designing, creating and testing their very own bio-based, biodegradable plastic or repurposing petroleum-based plastic in another form.

Comments: