Background – The Science
Nanotechnology is an emerging field of science that is the study of controlling matter on an atomic and molecular scale. Nanoscale refers to objects with at least one critical dimension between 1 and 100 billionths of a meter. The properties of matter that most of us learned in school do not always hold true for materials at the nanoscale. I felt like my world shifted as I learned about nanoscience because the proportional reasoning that I relied on from my math background, and the intensive properties of matter that I studied and taught in science classes, may no longer apply. Instead, there are size-dependent properties that apply to objects on the nanoscale.
To understand nanotechnology applications, it is helpful to understand the basic (unique) chemistry of nanomaterials. As we learned in high school Chemistry (and probably other science classes, too), the basic unit of matter is the atom. The size of an atom varies from element to element, but most atoms have a diameter of at least 0.2 nm. Atoms consist of a nucleus, which contains protons and neutrons, and orbitals (often referred to as "shells") which contain electrons. The number of (positively charged) protons determines what type of element it is; the atomic number on the Periodic Table is equal to the number of protons in the nucleus. Electrons are negatively charged and are equal in number to the protons in a neutral atom. Electrons are in constant motion in the orbitals outside of the nucleus; their exact location is never known, but there are locations where they are more likely to be at a given time (this distributed location of an electron is sometimes referred to as an electron cloud).
It is the number and position of electrons that determine what atoms do when they come in contact with other atoms – whether they react or not, and how. Some atoms, such as the Noble Gases like helium (He) and neon (Ne), do nothing; they are more stable as individual atoms because electrons completely fill all of their outer orbitals. Other atoms—such as sodium (Na), chloride (Cl), carbon (C), and oxygen (O)—interact with each other to form molecules. This interaction occurs by ionic, covalent, or metallic bonding.
Some atoms can easily transform to charged particles, called ions, because their outer orbitals are either nearly empty or nearly full. Atoms form positive ions by giving up typically 1or 2 electrons, or negative ions by accepting 1 or 2 additional electrons. Positive and negative ions join together with ionic bonds in a way that creates a neutral molecule. Sodium Chloride, table salt, is a classic example of a molecule formed with ionic bonds. Sodium (Na) easily gives up one electron to become the positive ion Na +. Chlorine accepts one additional electron to fill its outer orbital to become the negative ion Cl -. The two ions join together in a 1:1 ratio to form the neutral molecule NaCl. Other atoms "do not want to" (energy/stability-wise) completely give up or take in electrons, but would "be willing to" share electrons with another atom in the formation of a molecule. This sharing of electrons between atoms is called covalent bonding. In covalent bonds, the electrons travel back and forth between the two atoms so that each one has completely full outer orbitals some of the time. Ionic and covalent bonds are the most common types of bonding between atoms; however, a third type of bond, metallic bond, allows electrons to float "delocalized" among several adjacent metal atoms that are in a lattice arrangement.
Ionic and covalent bonds, which join atoms together into molecules, are examples of intramolecular forces. Molecules also experience affinity to one another: the forces holding the molecules together are called intermolecular forces. When the forces between molecules are weak, molecules move easily relative to each other: these molecules are stable in the gaseous state. Liquids have stronger intermolecular forces, and solids even stronger, preventing as much movement. There are two major types of intermolecular forces – hydrogen bonding and London dispersion forces. While intramolecular forces are always stronger than intermolecular forces, hydrogen bonding is the strongest of the intermolecular forces and accounts for some unexpected properties. For example, water
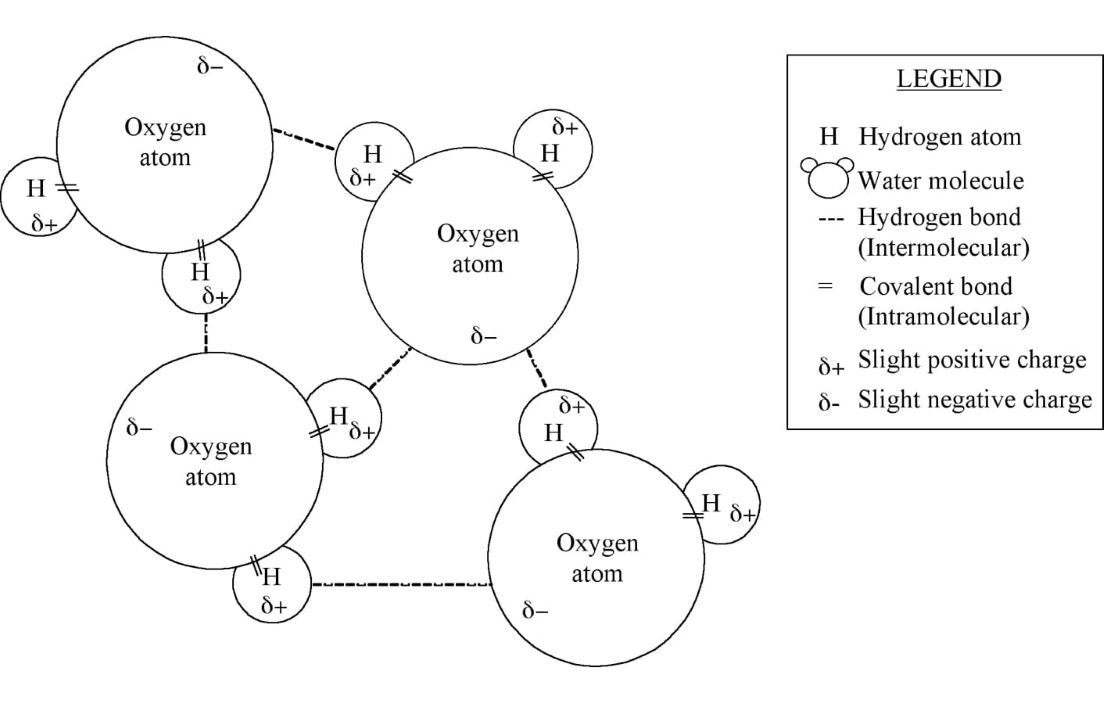
Figure 1 – Bonding in and between water molecules
has a high boiling point because its molecules (in the liquid state, of course) are held together by hydrogen bonding. Hydrogen bonds (refer to Figure 1) are formed between two polar molecules – molecules that have a partial positive region and a partial negative region because of an unequal sharing of electrons. Water is a polar molecule because the oxygen atom is so much larger than the hydrogen atoms that it has a stronger attraction for holding onto the single electron each hydrogen atom has to share. As a result, the electrons spend more time in the oxygen orbitals creating a partial negative region of the molecule, while the hydrogen atoms have no electrons in its orbital most of the time, creating a partial positive region. The intermolecular force is created by attraction between the partial negative region (oxygen) of the water molecule and the partial positive region (hydrogen) of another water molecule, thereby creating a hydrogen bond holding the water molecules together. London dispersion forces are weaker intermolecular forces between temporary polar molecules. Temporary polar molecules are created from fluctuations in electron density (location of electrons in the orbitals) or are induced to become polarized by other nearby polar molecules. London dispersion forces increase as molecular size increases because the electron cloud can become polarized more easily when electrons are farther from the nucleus.
These interactions between molecules lead to the properties of matter. There are macroscopic properties that include temperature, pressure, and viscosity that we can measure with common technology. On the other hand, measuring microscopic properties such as molecular size and shape, molecular velocity, and intermolecular forces would require sophisticated technology typically not available in high schools. Properties of matter are also split into two categories: intensive and extensive properties. Extensive properties such as mass, volume, heat capacity, energy, and entropy always depend on the amount of material present. For example, the larger the sample, the greater the mass and volume will be. Intensive properties, as I learned in school, were properties that do not depend on the amount of material present. Examples of intensive properties are color, temperature, density, pressure, viscosity, thermal conductivity, and electrical conductivity.
However, as I learned in this seminar, some intensive properties are size-dependent and may change when particles reach the nanoscale. For example, a chunk of gold metal is gold-colored but the color of gold nanoparticles (on the order of 10 nm, or 10 -8 m) ranges from blue to red to yellow to colorless depending on their size! Electrical conductivity and magnetism also depend on particle size. A material that is conductive at the macroscale (i.e. things we can see) may be a semi-conductor or non-conductor at the nanoscale depending on the level of confinement of the electrons in the material. 1 As an example, metals are conductive, since metallic bonds allow electrons to float with little confinement; therefore, less confinement of electrons allows for increased conductivity.
Probably the most critical size factor affecting properties of matter at the nanoscale is the surface area to volume (S/V) ratio. As an illustration, consider cutting a sugar cube multiple times. With each cut, there is more surface area exposed but the total volume (and mass) of sugar remains the same. We know from experience that the physical property of rate of solubility of the sugar cube will increase if we break it into smaller pieces: rate of solubility increases as surface area increases. Some other examples of the effect of the surface area to volume ratio are:
the adhesion property of powdered sugar ("sticking" to the sides) is greater than granulated sugar in a plastic measuring cup, the burning rate of a thick log is lower than the same mass of twigs, and the increased ability for the small intestine to get nutrients because of the millions of villi on the lining that increase its surface area. 2
Mathematically we know that increasing/decreasing all dimensions of an object by some factor (i.e. multiplication factor) will increase/decrease the cross-sectional area by that factor squared since area is a 2-dimensional measurement. Likewise, the volume of the object will increase/decrease by the factor cubed since volume is a 3-dimensional measurement. Since the area and volume change at different rates when the dimensions change, the S/V ratio also changes. To be more specific, as particles get proportionally smaller, the ratio of surface area to volume increases because we are dividing by a smaller number (the 2 nd object is a fraction the size of the first, and a fraction cubed is an even smaller number). As an example, consider two round balls of pizza dough, one with a 4-inch diameter and the other with a 2-inch diameter. The surface area of the larger ball (radius = 2 inches) is 4Π(2) 2 = 16Π in 2, and its volume is 4Π(2) 3/3 in 3 . The surface area and volume of the smaller ball (radius = 1 inch) are 4Π in 2 and 4Π/3 in 3 respectively. That makes the S/V ratio for the larger ball 3/2, and S/V for the smaller one 3. Since 3 is greater than 3/2, this example confirms that the surface area to volume ratio increases when the linear dimensions (radius, in this case) decrease.
In our Nanotechnology seminar we spent a significant amount of time discussing "why size matters," also the title of a book by John Tyler Bonner. We considered the relationship of weight versus strength in that if a person grew 10 times taller, then his weight would increase accordingly, but his legs would not be able to carry that much extra weight; the bones would not have enough strength to support him. By comparison, an elephant's legs are shorter and thicker to support its own weight, and ants can carry many times their own weight because of the strength of their legs relative to their body weight. One of the most intriguing concepts I learned in this seminar is that the effects of gravity versus molecular cohesion are hugely dependent on size; for this reason, gravity has a much greater effect on humans than it does on very small creatures. A fly, for example, can climb a vertical wall without falling to the ground because, for its insignificant weight, the molecular cohesion forces between the fly and the wall are more significant than the force of gravity. Of course nature's design plays a role; a gecko can climb a wall because of its small size (and weight) and also because it has lots of tiny hairs on its feet to provide increased surface area for even greater cohesion.
The element carbon deserves special attention: I will use carbon as the basis for my instruction in this unit. The atomic structure of carbon allows it to form four strong, covalent bonds with many different types of atoms, including four other carbon atoms. There are different forms of pure carbon, called allotropes, which have very different bonding structures and, therefore, different properties. The most common
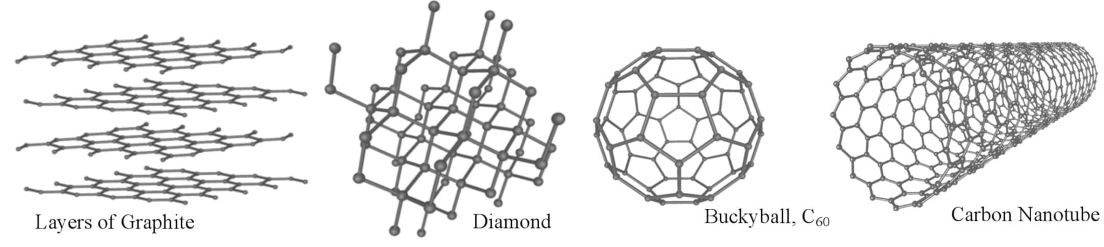
Figure 2 3 - Carbon Allotropes
forms of carbon are graphite, diamond, and carbon nanomaterials, called buckyballs (buckminsterfullerenes) and nanotubes. The strength of diamond comes from the tight arrangement of each carbon atom being bonded to four other carbon atoms in a tetrahedral shape extending to the edge of the sample. In contrast, graphite is softer because it bonds to only three other carbon atoms to form layers of hexagonal rings across a lattice, and the layers are held together by the relatively weak London dispersion forces. The "extra" electrons float as in metallic bonds. These floating electrons make graphite electrically conductive, whereas diamond is not. Buckyballs, discovered in 1985 by Harry Kroto, Richard Smalley and Bob Curl, 4 are spherical molecules formed by 12 pentagons and 20 hexagons that look like a soccer ball. The diameter of a buckyballs is approximately 1 nm! Whereas diamond and graphite structures can contain any number of molecules—because their structures are theoretically repeated—buckyballs contain exactly 60 carbon atoms, each bonded to three other carbon atoms. Their properties are not well-defined, except that they are able to bond on the surface with many other atoms and have the potential to trap smaller atoms on the inside. The discovery of carbon nanotubes was credited to Sumio Iijima in 1991. 5 Carbon nanotubes are basically graphite layers rolled into cylinders with a diameter of approximately 1 nm but have varying lengths. They come in different forms depending on their orientation 6 - imagine rolling chicken wire horizontally, vertically or on the diagonal to get different orientations of the wires. There are also single-walled and multiple-walled nanotubes, some with both ends open, and some with one end closed and rounded off. Nanotube properties are better defined than buckyballs. They are extremely strong (high tensile strength), conduct hot and cold, and can be either electrical conductors or semiconductors depending on their structure.
So, once we know about nanoparticles, it would be helpful to know how to produce them. One method is called "top-down" manufacturing, which involves starting with an object and removing part of it either mechanically or chemically (i.e. etching process) until we obtain material of the right size. Another production method is "bottom-up." Given the right conditions, nanoparticles will self-assemble, meaning that they will organize themselves into a stable orientation spontaneously. Self-assembly happens in nature all the time; the formation of cell membranes from phospholipids is one example. John Pelesko, in his book Self-Assembly: The Science of Things That Put Themselves Together, gives the example of a common cereal that self-assembles into hexagonal shapes when put into a bowl of milk, as long as the cereal pieces float, enough time passes, and perhaps the bowl is given a single shake. Self-assembly "provides a useful means for manipulating matter at the nanoscale." 7
Scientists continue to find new ways to exploit the unique properties of nanomaterials. As more sophisticated instruments, such as a scanning electron microscope, were developed, scientists were able to "see" nanoparticles. At the same time, nanotechnology is being used to make tools and instruments that can sense changes in and the concentration of things such as contamination or disease. In my readings, I found dozens of career area and industrial applications of Nanotechnology. The medical field, the dominant setting for our seminar, is using nanoparticles for drug delivery and its controlled release, medical imaging and cancer therapy. The computer and electrical industries are using Nanotechnology to increase RAM and data transfer rates, for robotics and to make transistors, conductive wire, energy-efficient light bulbs, and high-resolution displays. Other applications include genetically modified crops, paint with greater adhesion, composites of greater strength and less weight, fuel cells, cosmetics, cryptography, ink jet printer inks, and food. An annotated list of websites and books for learning more about these applications is given in the Teacher Resources and Bibliography sections of the Appendix.
While this unit is geared toward mathematics, I would be remiss if I ignored the fact that Biology is steeped in Nanotechnology. All living organisms depend on DNA, which is a nanomaterial because the diameter of the double helix is approximately 2.5 nm. It is the understanding of nanoscale biological processes that is allowing scientists/engineers to make progress in areas like the delivery of cancer drugs to targeted areas in the body. In addition, some of the research being done in the field is called Biomimicry or Biomimetics - the process of imitating what nature does, but under controlled conditions in the laboratory. Physics also relates to Nanotechnology in the study of energy, Brownian motion, entropy, enthalpy, viscosity and quantum mechanics. As much as I would like to, I don't have time during the semester to go into depth on the science of Nanotechnology because of the breadth of my required mathematics curricula. However, I can easily envision integrating units with Biology, Chemistry and/or Physics teachers in my building in the future.

Comments: