Appendix 5: How to Make Soap
Soap A: Teacher will demonstrate this soap making activity.
Purpose: To demonstrate how to make soap.
Materials: 20 grams lard or vegetable shortening (solid), 5 grams baking soda, water, hot plate, 20 grams of salt, cheesecloth, beakers, containers, molds.
Procedure: 1.Dissolve 5 grams of baking soda in 10ml of water.
2. Add 20 grams of lard to this solution.
3. Boil gently on a hot plate for 20 minutes. Can demonstrate temperature change here. Stir continuously while the mixture is boiling (stir so substance can mix thoroughly for saponification to occur). Can also illustrate the law of conservation of matter, based on the chemical equation.
4. Let the mixture cool, then pour into a plastic container and place on an ice bath for 5-10 minutes, stirring continously. (temperature change)
5. Dissolve 20 grams of salt in 25 ml of water to make a saturated solution. Add to the cooling mixture and stir. (this solution should be curdling now)
6. Gently take the container and pour through a cheesecloth until the container is empty. Drain any excess liquid. Pour the soap curdles in a mold and leave to set.
7. Test with litmus paper to determine acidity or alkalinity.
Explain saponification, solutions/saturated, temperature changes.
(Adapted from Prentice Hall, Science Explorer, Physical Science, pg. 138-139, 2002)
Soap B: Student's Soap
Purpose: To demonstrate how to make eco-friendly soaps
Materials: double boiler, glycerine soap base, essential oils, cocoa butter, fragrances, soap colorant, molds.
Procedure:1. Place the glycerine soap base into the top container of the double boiler and set the temperature on high to melt the glycerine until the moisture is remove, but not so that it forms bubbles.
2. Add the coloring to it, ensuring that the glycerine is evenly coated and absorbed the color.
3. Slowly stir in essential oils and fragrances.
4. Add melter cocoa butter, stir until the mixture is very smooth and even.
5. When it is completely bound together, slowly pour into molds and allow to set & harden.
For softer texture; add goat milk, whole milk, almond milk, etc. Students will use different oils and fragrances based on the results of their survey. Students will be required to measure and chart the temperature change every 3 minutes. Students will also illustrate using a chemical equation to indicate law of conservation of matter.
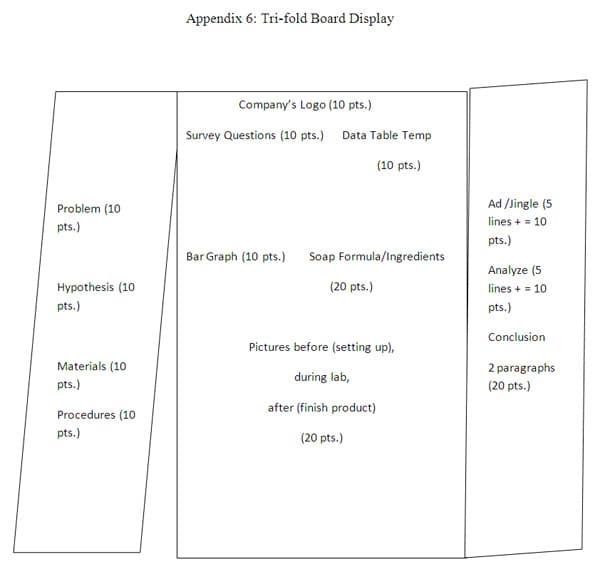

Comments: