Background Research
Where did soap originate?
The origin of the word soap is suggested to come from the Latin word sapo 3 stemming from a story about 3,000 years ago on Mount Sapo, near Rome, where the fat from the burnt sacrificial animals, mixed with the ashes from the altar, ran downhill over the clay soil, forming a slippery mixture. The women discovered that this actually helped to wash and clean their clothes, and so the story goes. The term sapo is a common derivative that is depicted in different languages, for example in Greek it is sapon, Hindi- Saban, French-Savon, Hebrew-Sabon, and Icelandic-Sapa. 3 On the other hand, the word "detergent" is derived from the Latin word detergee, which means to wipe or clean, hence the Latin de meaning off, and tarsus, meaning cleansing or heat, referring in modern times to synthetic soap. 3
Historians have traced the use of soap back to the 3 rd millennium B.C.E. to a Sumerian clay tablet discovered in the ancient city of Babylon from the Ur dynasty, with an inscription that stated, "With water I bathed myself, with soda I cleansed myself, with oil from the basin I beautified myself." 3 This marked humankind's history of using soap. The Celts called their combination of animal fat and plant ashes saipo, the Romans used mutton tallow and wood ashes to make their soap. 4 This process was officially named saponification, which is a reaction in which an ester is heated with an alkali, such as sodium hydroxide, producing a free alcohol and an acid salt, especially alkaline hydrolysis of a fat, or oil to make soap. 5 The process to make soap underwent various trials and errors from LeBlanc's soda ash, to Berthollet's use of chlorine to Tennant's concept using lime to form bleaching powder, to Gunther's synthetic detergent using castor oil. 4 The Crimean War of 1854-1857 resulted in Britain losing most of the military to infectious diseases. Florence Nightingale's rise to fame was attributed to helping Britain with the use of soap and instituting hygienic practices in nursing. This concept aided the Americans in their Civil War, which later propelled the manufacturing of soap into an industry. 4 The uses of soap varied depending on culture, location, and era, but mainly it was for agricultural, cleaning, washing, ceremonial rites, industrial, medicinal and personal uses.
What properties do soaps and detergents have? (similarities and differences)
Both soaps and detergents are used as cleaning agents. They are both surfactants; that is, they are surface active agents that change the surface or interface. For instance, if water touches a counter top, then both the water and the counter top are surfaces and the point where they touch is call the interface. Surfactants are classified according to their properties in water which can be ionic (electrical charge), anionic (negative charge), nonionic (no charge), cationic (positive charge) and amphoteric (either positive or negative charge) properties in water. Soap is an anionic (negative charge) surfactant. 7 One of the main differences between soaps and detergents is their behavior in water. A soap molecule is shaped like a tadpole with a head and a tail. The head mixes well with water, whereas the tail mixes well with grease and dirt. So, when you are washing, the soap molecules surround the dirt and break it up into smaller pieces so that the water can now wash it away. In hard water, minerals such as, calcium, magnesium and iron are dissolved in it, and form deposits called scum. Scum does not wash away easily because it is difficult to dissolve. Hard water does not allow for soap to lather well, and it will leave hair dull after washing. Hard water does not affect the cleaning action of detergent.
Acids, Bases and Salts
Another difference between soap and detergent is their sensitivity to acidic conditions. To understand the action of soap and detergent, we will review the pH scale to describe the characteristics of acids, bases and salts. Acids are substances that donate hydrogen ions, H +, to form hydronium ions, H 3O +, when dissolved in water. Acids taste sour and will turn blue litmus paper red. Acids can be very dangerous if not diluted with water. All acids conduct electricity, some more than others. Strong acids fully ionize when dissolved in water. This means their solutions have as many hydronium ions as the acid can possibly form. Acids range from 0 to 7, on the pH scale. Bases are substances that either contain hydroxide ions, OH &nd ash;, or react with water to form hydroxide ions. Bases taste bitter, are slippery and turn red litmus paper blue. Bases are also dangerous if not diluted with water. Potassium hydroxide, KOH, is a strong base and conducts electricity well. Potassium hydroxide will disassociate completely to form ions, when in water, KOH —> K + + OH -. Bases are from 7-14 on the pH scale. Potassium hydroxide (KOH) or potash is a very strong base, and is used in the manufacturing of soap, drain cleaners and bleach. Sodium hydroxide (NaOH) or lye is also a very strong base and is used to manufacture soap, drain cleaner, paper, and textiles. pH measures the concentrations of hydronium ion and hydroxide ion in a solution. A pH number or value will indicate how acidic or basic a solution is. In a solution with water, the more hydronium ions there are, the more acidic will be the solution, and the fewer hydroxide ions indicate the less basic the solution will be. A pH of 7 will indicate that the solution is neutral and is neither acidic or basic. 8 Neutralization is a reaction between an acid and a base leaving it neutral; so, it is neither an acid nor a base. Neutralization depends on concentration, volume, and the identity of the reactants. Sometimes the acids and bases react to form an acidic solution because the acid was the larger amount. The common product of neutralization is a salt, which is an ionic compound formed from the reaction of a positive counterion of a base and the negative counterion of an acid.
How is soap formed?
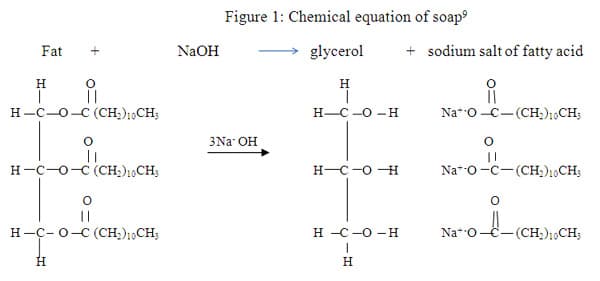
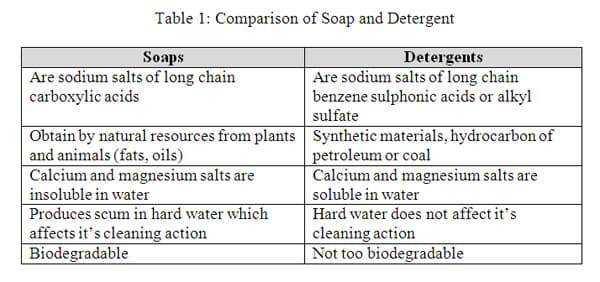
Soap is formed from the sodium salt of fatty acids in the hydrolysis of a fat as indicated in the equation above. We observe that the glycerol turns back into an alcohol and the fatty acid is turned into a salt due to the presence of the NaOH or (KOH). The properties of the soaps vary according to the type of fatty acids and the length of the carbon chain. Tallow or animal fats with 18 carbons will make a very hard, insoluble soap, whereas fatty acids with longer chains will be more insoluble. Coconut oils, on the other hand, have 12 carbons, which makes the resulting soap more soluble. 10 The common lipids for soaps are coconut oil, sunflower oil, palm oil, tallow, and olive oil. There are two types of soaps based on the action of saponification; they are sodium soaps, which are hard and used as hand soaps, and potassium soaps, which are soft and used as bath soaps. 1 0 Solubility is not the only characteristic of soaps. The polar and non-polar structure will determine how the soap gets rid of dirt. The long hydro-carbon chain is non- polar and hydrophobic (repelled by water), whereas the salt part of the soap molecule is ionic and hydrophilic (soluble in water). 7 Therefore, when soap is added to water, then the ionic-salt end of the molecule is attracted to the water and the non-polar end is repelled by the water as illustrated in Figure 2 below.
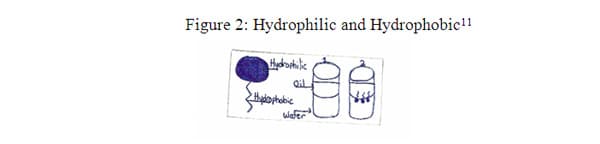
Drops of soap in water will then form a monolayer on the surface of the water, and the soap molecules will stand up. Surfactants are both lipophilic and hydrophilic, having dual affinity. They are classified according to the charge of the hydrophilic head. Most soaps, shampoos and personal cleansing bars contain an anionic surfactant.
How do soap and detergents clean?
Soaps are emulsifiers; this allows the oil and the water to mix, and keeps them from separating. Soap can dissolve in both oil and water. This makes soap a good cleaner. As an example, when someone washes their face with soap, the oil on the face is suspended in soapy water. Then by rinsing the face with water, the soap and unwanted oil is carried away and the face is now clean. So, when soap molecules are added to water, they clump together to form micelles, as illustrated in figure 3. These micelles are important to the cleaning properties of soaps and detergents. The negative ion in soap is made of long fatty acid chains and the positive ion is made of potassium or sodium ion. Because the soap acts as an emulsifier it will surround the droplets of oily dirt and the fatty acids will mix with the oil and the negative and positive ions will mix with the water. The resulting droplets of oil and dirt are surrounded by soap and are suspended in the water, and can now be rinsed away. 8 Figure 3, below, illustrates the action of the micelles as they clump and so a droplet of oil that can stay suspended in water because the charged ends of the soap dissolve in water and the uncharged ends dissolve in oil. 8
What environmental concerns are there with soaps and detergents?
There are some environmental concerns in the manufacturing of soaps and detergents. In the case of soap, the main idea is to arrange for safe transportation and containment of the raw material, as well as, keeping loss to a minimum. The oils and perfumes for soaps can spill but the drums are kept tightly sealed, and can be readily cleaned up. The perfumes are not flammable so that is not a concern. The acidity and alkalinity of the waste byproduct of soap manufacturing is constantly being checked to meet requirements. Loss is kept at a minimum and there are interceptors in place for emergency situations. In the manufacturing of detergents, there are two things to constantly monitor, that is dust control and volatile organic emissions. Dust is present with the transporting of powdered raw and complete detergents, and this can be dangerous. The dust level emissions should be below 50 mg m -3. 13
So, what are environmentally friendly cleaning products?
They are products that contain ingredients with less contaminants, carcinogens, phosphates, and petroleum. These are not biodegradable or if they are, they are slow, and therein become harmful to the environment, and other organisms, especially marine life. The sulphonic acid and nonionic detergents that are found in powder and liquid detergents are biodegradable. It is suggested that we choose cleaning products that are completely phosphorous and phosphate free (these are added to make water soft and more alkaline), and to avoid chlorine-based bleach, which produces dioxins, harmful to the inner body, and the environment. 13 Sodium citrate and sodium bicarbonate can be used to replace builders like phosphates. Another ingredient in cleaning products to avoid is surfactants that are made from petrochemicals (petroleum), because these are nonrenewable and slow to biodegrade. Surfactants like plant oil, preferably coconut, palm kernel and corn oil, are natural and easier on the environment. Antibacterial cleaning agents create anti-biotic resistant super germs and are to be avoided for health purposes. 13 Manufacturing plants suggest that eco-friendly soaps and detergents can make lesser use of chemical ingredients, non addition of additives like perfumes, color, brightening agents to decrease toxicity, minimal packing, and reduce or eliminate dyes and fragrances. Lastly, we are encouraged to carefully consume from companies who specialize in being respectful to the environment producing healthy, green products, as well as continuing to be respectfully vocal and assertive on the legislative level.

Comments: