Curriculum Content
Homeostasis
Homeostasis is the body’s ability to maintain a constant internal environment despite changing external conditions. Some of these specific factors include pH, temperature, and osmotic pressure. The idea of homeostasis is frequently brought up in middle and elementary classes although the process behind homeostasis is never really covered other than in the most shallow level. In reality, homeostasis is maintained through a set of body surveillance protocols including those of the endocrine system. The body uses both the nervous system and the endocrine system to maintain the conditions of the body.
Unlike how it is normally portrayed, homeostasis is not a constant state of the body tissues. They body is constantly undergoing fluctuations within their various physiological conditions. The role of homeostatic regulatory systems is to ensure that the levels of the body maintain themselves within a very narrow range—never too far from the optimal conditions of the body. This is crucial because a failure to maintain homeostasis could result in a multitude of detrimental conditions, some of which I address later.
There are a many homeostatic regulation examples in humans and other mammals, but most operate under the same principle. A sensory receptor is stimulated by a particular condition. This sensory receptor sends a signal to an area of control, which interprets this signal and decides whether to signal an effector to change the internal environment. In the next section of this paper, we discuss the endocrine system and the chemical indicator molecules called hormones used in this process.
An easy way to think about homeostasis is to consider a common thermostat. A thermostat is set to a desired temperature and functions to maintain the room at that temperature. However, this temperature is actually an average of the actual temperatures that the room cycles through during the day. If there are a number of people in the room, or if the outside temperature increases, the room temperature also increases. When the room temperature increases, a sensor in the thermostat recognizes this as outside the normal range and triggers the air conditioning to start pumping colder air into the room. When the temperature reaches normal, the thermostat sensor sends another signal to the air conditioner to turn off. The body is constantly sampling the conditions of the body and comparing them to “normal”. If the body starts to deviate, there are systems in place to step in and counteract these changes, restoring balance. This homeostatic example is a representation of a negative feedback loop, which is discussed later in this curriculum unit.
The important take away message about homeostasis is that our bodies work optimally in specific conditions. When those conditions are disrupted, then sensors are able to identify this change and take measures to correct it. When this feedback mechanism is disrupted—if the thermostat is broken—and the physiology of the body continues to move out of alignment, it is likely that there will be negative consequences.
The Endocrine System and Hormones
One of the reasons why endocrinology is difficult to study is because, unlike most organ systems that are found connected physically and are in close proximity to one another, the endocrine system is a diffuse series of mostly unconnected glands distributed throughout the body. There are several glands that are found in both sexes and glands that are responsible for each sex. This section describes the major function of each endocrine gland and several key hormones that that particular gland is responsible for. Gland positions are described using standard anatomy directional terms. For a quick primer on these terms, please reference the anatomy primer that is referenced in the Teacher Resources at the end of this document. Figure 1 also has a directional term compass, which may be useful.
In addition to the written description of the endocrine glands, see Figure 1 (Endocrine System Diagram) as to the placement of each. Table 1 (Endocrine Glands, Hormones, and Target Tissues), which is located in the appendix, also includes a brief overview of the major hormones associated with each endocrine gland. For an in-depth analysis of the various endocrine hormones, please reference the section titled Teacher Resources.
Glands of the Brain
Pituitary Gland: The pituitary gland is located at the base of the brain, just inferior to a second endocrine gland called the hypothalamus. The pituitary gland is found anterior to the mid-sagittal line, proximal to the olfactory bulbs of humans. Even though it is a small gland—only about the size of a pea-- the pituitary gland is considered to be the “master gland” of the body. It is trisected into major regions. The anterior pituitary gland is responsible for a multitude of puberty developments, sexual maturation, and the control of growth. It is responsible for the stimulation of the thyroid, adrenal, and sexual glands. This region is also responsible for the stimulation of breast tissue to produce milk. The intermediate lobe is responsible for the release of a precursor hormone that stimulates melanocytes, which produce melanin. The posterior region of the pituitary gland is responsible for osmoregulation. It is also responsible the production of the hormone oxytocin, one of the hormones that stimulates a positive feedback loop resulting in the repeated and strengthening contractions of the uterine walls that leads to childbirth. This cycle in particular is addressed in detail in the section called Positive and Negative Feedback Loops.
Hypothalamus: The hypothalamus is located superior to the pituitary gland, to which it is connected by a very small bundle of nerves called the infundibulum. While the pituitary gland is credited as being the “master gland” of the body, the hypothalamus controls a multitude of essential autonomic and behavior functions. For example, this section of the brain has a role in the regulation of temperature, thirst and hunger, sleep, sex drive, and other behavioral functions. This gland works together with the pituitary gland and other endocrine glands to control these functions.
Pineal Gland: This very small gland is located posterior to the hypothalamus, just below the corpus callosum. The pineal gland was once regarded as the body’s “third eye” or as the “seat of the soul”1. The pineal gland does play a role in the control of body functions. This gland produces melatonin and helps to modulate the sleep cycle and circadian rhythm. There are also some indications that that the pineal gland may play a role in reproductive development although the exact mechanisms or role it plays are not quite understood.
Glands of the Torso and Abdomen
Thyroid and Parathyroids: The thyroid and parathyroid glands are found anterior to the trachea, surrounding the larynx. The thyroid gland is responsible for the regulation of metabolism through the use of triiodothyronine (T3) and thyroxine (T4). This gland also produces calcitonin, which is responsible for the maintenance of calcium levels in the blood stream. This gland is affected by the release of metabolic hormones by the pituitary gland and the hypothalamus.
The parathyroids are four small endocrine glands located immediately posterior to the left and right lobes of the thyroid. They are responsible for the regulation of phosphate and calcium levels in the body. Parathyroid hormone works antagonistically to calcitonin released by the thyroid, and promote the reabsorption of phosphate by the kidneys.
Adrenal Glands: The adrenal glands are small, triangular glands that sit immediately superior to the kidneys. The adrenal cortex is the outer layer of the adrenal gland. This layer produces three important groups of hormones: glucocorticoids, mineralocorticoids, and gonadocorticoids. Both glucocorticoids and mineralocorticoids are responsible for maintaining blood levels of blood glucose and sodium, respectively, while gondocorticoids help to regulate levels of sex hormones. The adrenal medulla is responsible for the secretion of two other hormones—epinephrine and norepinephrine—which are responsible for stimulating sympathetic nerves during stress.
Pancreas: The pancreas is located on the right side of the abdominal cavity, posterior to the stomach, attached to the duodenum through the pancreatic duct. This endocrine gland is responsible for the regulation of blood sugar levels. The islets of Langerhans contain tiny clusters of beta cells, which secret insulin, and alpha cells, which secrete glucagon. These two hormones work together to lower and raise blood sugar levels in the body.
Reproductive Endocrine Glands
Ovaries: Ovaries are responsible for producing ova in females. They also produce two important sex hormones—estrogens (estradiol, estrone, and estriol) and progesterone. These hormones work to produce secondary sexual characteristics, and maintain the health of reproductive systems. Progesterone works to prevent uterine contractions, protecting embryos as they form in the uterus.
Testes: As with the ovaries, testes are responsible for the development of secondary sexual characteristics in males, healthy sperm production, and the maintenance of bone density and muscle mass.
Non-Traditional Endocrine Structures and Their Hormones
In addition to the organs listed above, which are considered as traditional examples of the endocrine system, there are also several other organs that release hormones of their own. For example, the stomach is responsible for the production and secretion of several hormones including ghrelin and gastrin. The purpose of the hormone ghrelin is twofold: it plays a role in triggering hunger and also works as a promoter for growth hormone release. Gastrin increases the release of acid secretion.
Even bones play an endocrine role. They release a hormone called osteocalcin, which plays several important roles. First, it is responsible for the healthy development of bone by controlling the deposition of the minerals. This hormone also has several secondary roles including increasing the release of insulin, signaling for the replication of beta cells, and decreasing fat deposits in adipose tissue.2 These secondary roles of osteocalcin make it a prime research target for the control of diabetes.2
Another example of a non-endocrine organ that has endocrine function is the thymus. The thymus is a lymphoid organ that plays a large role in the immune system. This organ is found anterior to the heart, posterior to the sternum in a region called the mediastinum. This gland releases an important hormone called thymosin, which is responsible for the development of T cells. Interestingly, this gland is greatly reduced as a person ages, and upon maturation is replaced by adipose tissue.
As researchers develop a better understanding of the body, more of these non-traditional endocrine functions are being discovered and the pathways of their hormones mapped out. This adds another level of complexity to an already difficult system, which I believe is part of the reason why most secondary school anatomy and physiology curriculums only gloss over the endocrine system.
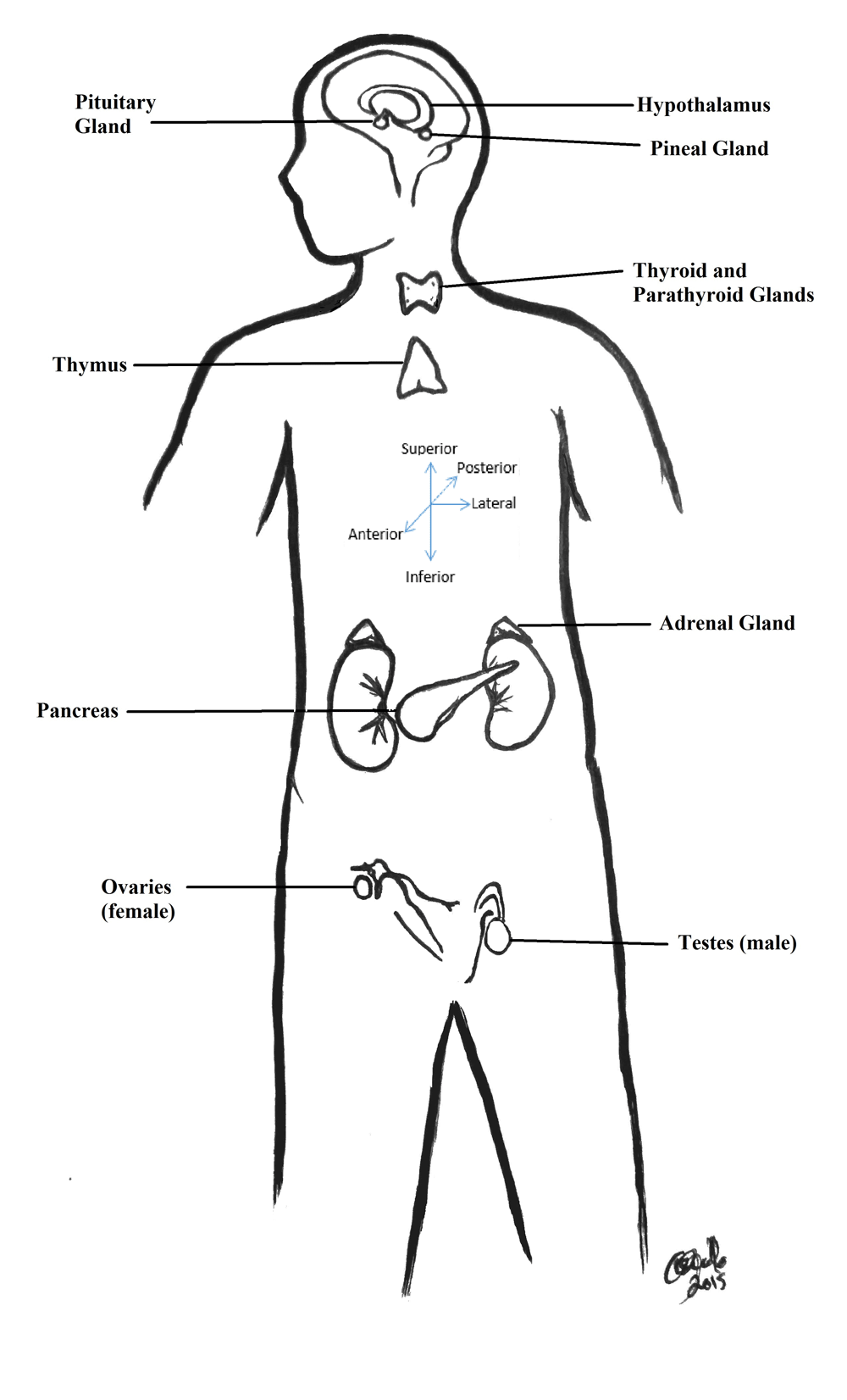
Figure 1. Distribution of Major Endocrine Glands in the Body.
This illustration represents the placement of endocrine glands around the body. Also included on this diagram is a basic analysis of anatomy directional terms that have been used to describe the gland positions.
Hormones and Target Tissues
A single endocrine gland may be responsible for releasing multiple hormones, which affect multiple types of tissues far away from the site of release. These glands release hormones directly into the blood, unlike exocrine glands that secrete their products through specialized tubes called ducts. The blood carries the hormones away from the endocrine gland and throughout the rest of the body.
In order to properly do their job, hormones need to be able to recognize target tissues. This process is mediated by receptors. Receptors are small protein structures located on the cell membrane or inside of the cell with ligand bonding sites that allow it to interact with complimentary molecules. Receptor protein-hormone interactions are crucial for the activation of tissues and relies on complimentary shapes bonding. There are many different compounds that pass over a receptor protein at any given moment, however the molecular structures may not be a perfect fit, similar to the lock-and-key model use to describe enzymes. In this case, the hormone is a key looking to unlock a specific door. If the shape of the ligand does not fit, or if the key does not fit the lock, they disengage. When the shapes are complimentary, then an action can occur—in the case of the hormone, an effector signal can be triggered in the target tissue. In the ligand-hormone interaction, the receptor latches on to the passing hormone and is less likely to release it immediately. When the hormone sits in the receptor, it triggers a reaction inside of the tissue specific to that particular sensor.
The endocrine system has several additional nuances that it must address in order to ensure that there is ample control over the various target tissues. First, each individual endocrine gland makes multiple hormones. Second, one hormone can affect more than one target tissue. For example, adrenaline—a hormone that is released by the adrenal glands—has target tissues in the cardiovascular, digestive, and respiratory systems. Third, a single target tissue can be affected by multiple hormones, released by more than one endocrine gland. The complexity in the delivery of the correct hormone to the correct target tissue is controlled by receptors.
Positive and Negative Feedback Loops
Hormones are regulated through a series of feedback mechanisms, often with one hormone triggering a second and so on. There are two types of feedback loops that students must understand in order to understand how the endocrine system functions: negative and positive feedback loops. Negative feedback loops are, perhaps, the most easily understood of these two systems. In a negative feedback loop, there is an initial change in the body, which moves the body away from “normal”. The body reacts with an antagonistic reaction, which counteracts the change in the body. This response returns the environment back to normal, which then switches off the feedback loop. This type of feedback is seen in the regulation of blood sugar levels and is akin to the way a thermostat regulates the temperature in a room. In this sense, negative feedback loops act as a buffer to change, preventing a movement too far from equilibrium. For an example of a negative feedback loop using insulin, see Figure 2 below.

Figure 2. Negative Feedback Loop for Blood Sugar Regulation.
In the negative feedback loop that is responsible for regulating blood glucose levels, the hormone released will work to reestablish a set point. Once homeostasis is restored, the hormone is no longer released which shuts off the feedback loop. The green portion of the feedback loop represents the process for lowering blood glucose levels while the blue portion of the feedback loop represents the process for raising blood glucose levels. These two cycles work together to maintain balance.
Positive feedback loops produce amplifications. Instead of being ‘turned off’ by the product of the loop, this product perpetuates the change from the “normal” condition, moving the environment further from equilibrium. While this seems to counter act the idea of homeostasis, this process is actually vital to several human functions. Oxytocin, the hormone responsible for stimulating uterine contractions during childbirth, is controlled through a positive feedback loops. This is why contractions start small and far apart and intensify as time goes on until the child is born. For an example of a positive feedback loop featuring oxytocin, please see Figure 3 below.
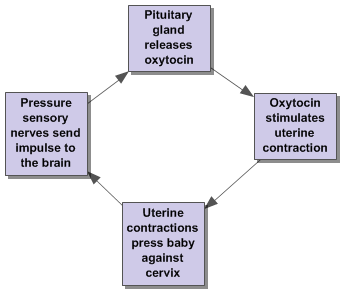
Figure 3. Oxytocin Feedback Loop.
In the oxytocin feedback cycle, the release of the hormone propagates uterine contractions which, in turn, signal for more oxytocin. This amplification of the signal continues until the child is born, which reduces pressure against the cervix and stops the cycle.
Feedback isn’t as simple as I make it seem—often times these loops are inter-connected and involve more than one mechanism or more than one controlling hormone. Take for example the hormones involved in blood sugar regulation. Most units on endocrinology use the insulin feedback loop as an introduction to negative feedback mechanism because it appears to be relatively simple. As blood sugar increases, beta cells in the islets of Langerhans of the pancreases release insulin, which triggers the uptake of blood sugar into hepatic storage and glucose muscle reserves. This decreases the amount of sugar in the blood, returning it back to normal homeostatic levels, and turning off insulin production and release by the beta cells.
However, this mechanism is much more involved than this simple loop. Multiple hormones also play a role in blood sugar maintenance. The hormone osteocalcin, which was mentioned above in non-traditional endocrine tissues, is also released in order to increase insulin levels.2 At the same time, the hormone somatostatin works to reduce the expression of the hormone glucagon by pancreatic alpha cells.3
When insulin production is reduced, the body cells move from storage mode to gluconeogenesis, but this again is not the whole picture. Glucagon, the hormone previously mentioned, is created by pancreatic alpha cells. It causes the release of glucose from glycogen storage and also triggers other body tissues to convert their amino or fatty acids into sugar residues. This process is enhanced by cortisol, released by the adrenal cortex, which in turn is amplified by the release of adrenocorticotropic hormone from the anterior pituitary gland.4 These two hormones, along with growth hormone from the pituitary gland, antagonize insulin and increase blood sugar. This brief summary does not take into account other hormones such as epinephrine and thyroxine, which also play roles in the maintenance of blood sugar levels.5
Multiple pathways, organs, and hormones can play a role in supporting the simplest of metabolic functions. Hormones can work synergistically to amplify an effect, and also work antagonistically to turn it off. The complexity in this system can also be explored by looking at the hormone somatostatin. As previously mentioned, this hormone reduces the release of glucagon, a hormone that is released by the pancreas in order to convert glycogen storage of sugar into usable glucose. This same hormone can also inhibit insulin production, preventing the reduction of sugar in the blood and interfere with the effects of growth hormone in the anterior pituitary gland.
The control of blood sugar also illustrates another important aspect of the endocrine system. As I previously mentioned, the insulin-glucagon cycle is only one of the pathways that is responsible for controlling blood sugar. Sugar regulation in the body is very important because levels that are too high can cause damage to tissues and tissues that are too low can also have serious physiological consequences. Since the control of blood sugar is so important to maintaining homeostasis, there are multiple pathways that are responsible for ensuring that blood sugar is kept at the correct level. This redundancy is an example of one of the control mechanisms that the endocrine implements in order to ensure maintains of homeostasis.
Another interesting example of the intricacy of endocrine feedback mechanism regulation occurs with luteinizing hormone (LH). During menstruation, the ovaries release small amounts of estrogen into the blood. Estrogen triggers the hypothalamus to release gonadotropic-releasing hormone (GnRH), which triggers the release of LH from the anterior pituitary gland. This hormone causes more estrogen to be released from the ovary6. This leads, ultimately, to a positive feedback loop as each of these hormones continues to amplify the release of the others in this cycle. LH is responsible for the formation of the corpus luteum, which is produced by ovulation. When the corpus luteum develops, this signals the release of progesterone, which inhibits GnRH which in-turn releases LH, turning the cycle off via a negative feedback loop.6
However, estradiol can also cause the pituitary gland to release less GnRH, which in turn inhibits the release of LH and other gonadotropic hormones. In essence, an increase in the specific estrogen estradiol actually prevents the release of LH and turns off the positive feedback cycle previously explained.7 During a certain point in the menstrual cycle, the amount of estradiol is reduced, turning off the inhibitory effect and allowing the cycle to switch to the positive feedback loop, which leads to ovulation.
Diseases of the Endocrine System
When the body fails to maintain homeostasis there are biological consequences. This section will only explain a few examples of endocrine related diseases that I find to be interesting. This brief survey is merely an example that can be used with students to demonstrate how the over or under production of specific hormones can lead to physiological problems.
I plan on using the following examples to illustrate how a variety of disease can happen by a disruption in the endocrine system. For example, both acromegaly and pituitary dwarfism stem from an error involving the release of growth hormone. I plan to use these two particular diseases to demonstrate what happens when there is too much or too little of the same hormone. Other diseases like Cushing’s syndrome or diabetes insipidus demonstrate how diseases of the endocrine system can imitate other common diseases like obesity and diabetes mellitus. I have listed several diseases below that I feel are of particular interest to my students and would serve as a strong introduction of how hormone disruption plays a role in disease.
As part of their coursework, students have the opportunity to explore the impact of a hormone, of their choosing on the body, and what happens when this particular hormone is disrupted. I highly recommend choosing examples that are of interest to students in your own classroom when presenting this portion of the unit.
I have also made the decision not to include diabetes as one of my examples in this unit. If you are interested in using diabetes mellitus as one example of an endocrine system disruption, I highly recommend you read the unit by Pricilla Black entitled “Diabetes and the Navajo Nation” from the same volume of curriculum units. Her unit is included in the Teacher Resources below and, while written for a different grade level, contains a lot of helpful background information and activities that can easily be scaled up for a high school classroom.
Acromegaly
Acromegaly is a disease that occurs when there is an overexpression of growth hormone. Growth hormone is released by the pituitary gland and is essential to the healthy lengthening of bones and development of muscles. Under normal circumstances, it is released during childhood and puberty as the body goes developmental changes. In adults, growth hormone is responsible for the maintenance of body structures and the regulation of blood glucose levels. Bursts of growth hormone are released usually during periods of sleep and stress as the body heals and repairs. The inhibitory hormone somatostatin, as previously mentioned, is responsible for reducing the release of growth hormone.
When growth hormone is released in excessive quantities or for greater than normal periods of time, there is a characteristic thickening of bones in the hands and face, and a thickening of vascular tissues including the heart in a condition called acromegaly. In most individuals, acromegaly occurs in adults after growth has already finished but in rare cases, it can occur in children. When this happens during the developmental phase, it results in increased long bone development and thickening in a secondary condition called gigantism. Excessive height can also lead to additional problems including joint pain, hypertension, and sleep apnea. André René Roussimoff, otherwise known as the professional wrestler André the Giant, was well known for his acromegaly. By the age of 12, André was 6’3” tall and near 240 lbs.8 At the peak of his career, he was a towering 7’4” and almost 500 pounds.8 This incredible height and stature resulted in numerous health problems including cardiomyopathy, heart problems, and difficulties healing from injury.8
Growth Hormone Deficiency
The opposite of acromegaly, growth hormone deficiencies, result from an under-secretion of growth hormone. Growth hormone deficiency can develop in several different ways. Congenital growth hormone deficiencies usually happen when the pituitary gland does not properly develop.9 This leads to a reduction of GH. This condition can also be acquired in childhood or adulthood in various ways. A severe trauma, or a severe acquired illness such as meningitis, may reduce function of the pituitary gland. Tumors may also cause a suppression of pituitary function.
Growth hormone deficiency isn’t usually diagnosed until the child starts to go through the early stages of puberty. Characteristics usually include a chubby, small face and body build. Long bones develop slowly and eventually taper off. Puberty might be delayed or might not occur depending on the underlying cause of the pituitary damage.9 Diagnosis of this disorder can be confirmed by comparing the size of the bones in a child’s hand to the expected size for their age. Bones are a good indicator of the age of an individual. In children, bones have epiphyseal growth plates that are areas for mitosis and bone elongation. As a child goes through puberty, these zones start to solidify. By comparing the size of the child’s bones to the expected size for a child at that age, doctors are able to look for deviation. If a child’s bones are two or more years behind the expected chronological age development, it is likely that there is a suppression of GH.9 This can be confirmed with blood tests. Growth hormone deficiency results in pituitary dwarfism. This particular form of dwarfism results in a proportioned, smaller stature.9
There are several treatment options for children who are exhibiting GH deficiency. Usually, doctors recommend regular GH shots, which can be administered at home, to replace the missing hormone. Children who are undergoing this treatment are required to take shots through puberty to ensure proper development.
In adults who have already reached their full height, acquired growth hormone deficiency might lead to a decrease in bone density and muscle mass. Adults may also experience fatigue and forgetfulness. 10 Hormone replacement therapy is also useful for reducing the symptoms of acquired GH deficiency.
Cushing’s Syndrome
Cushing’s syndrome, named after physician Harvey Cushing, was first characterized in 1932. 11 Patients with Cushing’s usually have hypertension, glucose intolerance, and osteoporosis. Cushing’s syndrome often corresponds with a gradual increase of weight and also abnormal fatty deposits between the shoulders or in the face. Many also display hirsutism, purple striae, and emotional changes.11 While many of the characteristics of Cushing’s syndrome mimic those of typical obesity, the underlying causes are different.
In Cushing’s syndrome, the patient is experiencing an over-production of the hormone cortisol. Cortisol is produced by the adrenal cortex. It is responsible for many different functions, including gluconeogenesis, immune and anti-inflammatory responses, and blood pressure through the control of vasoconstriction. Usually, cortisol is only released in normal pulses following the circadian rhythm or in situations of extreme stress. In Cushing’s syndrome, cortisol is released in excess of normal conditions.
Cushing’s syndrome can occur through a variety of means. The adrenal glands themselves are controlled by the pituitary gland. A tumor in this gland can cause an overexpression of adrenocorticotrophic hormone (ACTH), which can cause the adrenal glands to release excessive amounts of cortisol. This presentation can usually be identified by elevated serum ACTH and cortisol. This is the most typical cause of Cushing’s syndrome—about 80% of patients who exhibit Cushing’s have elevated ACTH.11 Certain lung cancers can produce spikes in ACTH, which may also cause the symptoms of Cushing’s syndrome. A second cause can be a tumor in the adrenal glands themselves. If this is the case, a blood test may show elevated cortisol, but not elevated ACTH. In fact, there are usually very low levels of ACTH in adrenal-tumor caused Cushing’s, since high levels of cortisol usually have an inhibitory effect on the pituitary gland (by the principle of negative feedback!). 12
As previously mentioned, blood tests are often used to identify the specific composition of the elevated hormones. These blood tests are usually confirmed with the use of a CT scan or an MRI of the area suspected to host the tumor. Once the diagnosis is confirmed, treatment can begin. If the tumor is in the pituitary gland, it is usually treated with radiation and surgically removed. Adrenal tumors are also surgically removed. After the tumor is removed, it is likely that the patient will need to take replacement hormones, since it is likely that hormones may now be under expressed.
Diabetes Insipidus
Diabetes insipidus is unlike diabetes mellitus (type 1 and type 2). In diabetes mellitus, there is a problem with insulin—either it is not produced in sufficient quantities (type 1) or cells in the body stop responding to insulin (type 2)—which prevents the normal uptake and regulation of sugar. In diabetes insipidus (DI), the main problem is the unusually high excretion of water by the kidneys. Antidiuretic hormone (ADH), which is released by the hypothalamus and stored by the pituitary gland, is responsible for keeping the water levels in the body correct. 13 This hormone works together with the kidneys to control the volume of water that is reabsorbed.
In DI, the body cannot retain water and the kidneys excrete large volumes of it as dilute urine. There are two main causes for the development of DI—either damage has been done to the hypothalamus and pituitary glands or there is a problem with the small tubes in the kidneys called nephrons which are responsible for the collection of water. As a result, other symptoms of DI can include excessive thirst, fatigue, weight loss, bladder control problems, and the production of between 3-15 liters of dilute urine a day.13
Since DI shares several of these symptoms with diabetes mellitus, diagnostic testing is important for distinguishing between these diseases. DI is not a “sugar” sickness, so blood glucose tests that are normally used for diabetes will not provide expected results. Urinalysis is a useful diagnostic test, and will usually demonstrate a low urine specific gravity or the concentration of solutes in the urine.13
Treatments for DI depend on the underlying cause for the symptoms. If there is an underlying endocrine problem, ADH replacement treatments can help the body retain additional fluids, restoring balance in the body.
Hashimoto’s Thyroiditis (Chronic Lymphocytic Thyroiditis)
While there are many forms of thyroiditis, Hashimoto’s thyroiditis is the most common. This autoimmune disease affects nearly 14 million people in the United States.14 This form of thyroiditis occurs when the body’s immune cells attack healthy thyroid tissue. This causes the tissue to become inflamed and lowers the production of thyroid hormones.
Hypothyroidism is often attributed to low levels of thyroxine. Thyroxine is a precursor hormone to T4. It is responsible for maintaining metabolic rate and body temperature regulation. As such, common symptoms of Hashimoto’s thyroiditis include fatigue, feeling cold, weight gain, dry or brittle nails and hair, constipation, and forgetfulness.14 These symptoms usually develop slowly. It may take several years for a patient to be diagnosed, since many patients ignore their symptoms.
A second major complication in Hashimoto’s thyroiditis is the development of a goiter. If the immune system attacks the thyroid over enough time, the thyroid swells into a large lump. It is possible for goiters to be seen pressing against the anterior tissues in the neck. While the goiter itself might not cause pain, it can put pressure on the esophagus and trachea, reducing their functions. 15
Hypothyroidism is often diagnosed using a thyroid stimulating hormone (TSH) blood test. This test measures the amount of TSH released by the pituitary gland. If TSH is overexpressed, it is likely that the thyroid is not producing adequate amounts of thyroxine. 16 A secondary test called anti-thyroid antibody test can be used to differentiate between Hashimoto’s thyroiditis and other forms of hypothyroidism. Hashimoto’s thyroiditis is characterized by its autoimmune function. As such, the immune system produces large amounts of thyroid antibodies. Anti-thyroid antibodies only react in the process of these thyroid antibodies. 17
Hashimoto’s is treated primarily by using thyroxine supplements. Pills are taken daily to increase the amount of thyroxine in the blood. Doctors need to regulate the dosage, since an overdose of thyroxine can also have complications, including osteoporosis and arrhythmia. 18 Iodine and calcium supplements are also recommended to help combat some of the other side effects of low thyroxine.18
Polycystic Ovary Syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is characterized by small, fluid filled sacs on the ovaries. These cysts cause a number of symptoms including pelvic pain, irregular menstrual cycles, and infertility. Other symptoms of PCOS are hirsutism, acne, and dark, flakey patches of skin. Some women can have excessive weight gain around the stomach and male pattern baldness in addition to their other symptoms. 19
While the exact mechanisms behind the formation of POCS are unknown, many women who exhibit this disease have excessive testosterone. Even though testosterone is considered to be a male hormone, it is made in the ovaries of women to help maintain bone density and muscle mass. During the process of oocyte maturation in the ovaries, follicle cells swell up with fluid.19 During normal ovulation, these follicles burst and allow the oocyte to travel towards the uterus. In a woman suffering from PCOS, these swollen follicles do not burst, but collect within the ovaries. Overexpression of testosterone in females can cause many of the symptoms seen in PCOS, including these cysts.
In addition to increased testosterone, many women with PCOS also have insulin resistance, preventing adequate regulation of blood glucose.19 In cases with insulin resistance, additional insulin is released from the pancreas but does not result in a change in blood sugar. This positive feedback loop can lead to increased hunger, since sugar isn’t properly used in metabolism, and increased testosterone.
Treatments of PCOS are aimed at treating symptoms. Since PCOS can manifest in a variety of symptoms, doctors often prescribe specific treatments aimed at lifestyle management and treatments similar to those for Type 2 diabetes are often used to reduce insulin related complications.19 Birth control medications, which include progesterone pills, help to mitigate many of the complications of high androgen hormones, including acne and extra hair growth.19 Additional treatments are available to help women with PCOS conceive.
Low Testosterone (Hypogonadism)
Testosterone, an androgen hormone produced by the male testes, is crucial to the proper development of secondary sexual characteristics formed during puberty. In adult males, it is responsible for helping with sperm production and libido, as well as the maintenance of muscle and bone health. Healthy adult males usually experience a fluctuation of testosterone throughout the day, ranging between 300-1000 ng/dL. 20
If males are exposed to lower levels of testosterone, they may experience several types of symptoms. Usually, there is a decrease in sperm count, impotence, and the development of breast tissue. Sustained low levels of testosterone may also decrease muscle mass, bone density, and cause symptoms seen in menopause like irritability and hot flashes. 21
There are two main types of hypogonadism—primary hypogonadism and secondary hypogonadism. In primary hypogonadism, the testicular tissue is damaged. This can result from an injury to the testicles including testicular cancer, Kleinfelter Syndrome, and undescended testicles.21 In all of these cases, damage or disruption of the testicles results in lower amounts of testosterone being produced.
Secondary hypogonadism results from a problem in either the pituitary or hypothalamus. Since these endocrine glands are responsible for releasing gonadotropic hormones, a disruption in these tissues may result in reduced expression of testosterone.21 Pituitary tumors can be one cause of secondary hypogonadism. There has also been a link between type 2 diabetes and an increased risk of developing secondary hypogonadism.
Levels of testosterone can be checked through blood tests, so this is the first procedure used to identify low testosterone levels. If low levels of testosterone are confirmed, physical examinations as well as CT or MRI scans can be used to identify the cause of low testosterone.20 If a tumor is present, it is removed through surgery.
One of the common treatments for this disorder is to use testosterone supplements. There are several ways that testosterone can be administered. One method is a deep tissue injection of testosterone every two weeks. This is a slow release form of treatment and is often considered to be the least expensive option. Testosterone gels can also be used, though the must be applied daily. In these treatments, testosterone is absorbed transdermally. Buccal medications are also available. These small tablets are inserted between the gums and lip and slowly release testosterone into the bloodstream. 22
Medical Interventions
Hormone Replacement Therapy
Usually when hormone replacement therapy (HRT) is discussed, it is connected to the use of estrogens in order to ease the symptoms associated with menopause. Menopause is caused by the senescence of ovary reproductive function and the cessation of the majority of estrogen production. 23 In addition to relieving the hot flashes and vaginal dryness that often accompany menopause, estrogen also reduces some of the calcium loss in bones. While HRT relieves many problems, it can also lead to endometrial cancers due to excessive cellular build-up of cells lining the uterus.23 Interestingly enough, endometrial cancers are reduced in women who take estrogen-progesterone combination HRT because progesterone encourages the endometrium to shed.
Many of the diseases that I discussed in the previous section demonstrate the use of supplemental hormones as a form of treatment, especially in diseases such as hypogonadism where a hormone is under-expressed. For example, Hashimoto’s thyroiditis symptoms are often alleviated using thyroxine injections. The use of synthetically created hormones or through farmed hormone sources have greatly reduced the impact of many devastating conditions.
I have already discussed the use of growth hormone in children with GH deficiencies in order to help with the proper development of bone and muscle. A 2013 study performed by Moreau et al demonstrates an unconventional use of GH in the improvement of memory and attention in patients who have traumatic brain injury. Increased GH boosts neurotransmitters which assist in cognitive functions. 24
Abuse of Hormones
Since we are a magnet school with a health science and sports medicine focus, the topic of growth hormones and steroids as athletic enhancement supplements is frequently brought up in debate. Some students feel that, since they are natural compounds already made by the body, application of these hormones to improve natural athletic ability should be allowed. Other students agree with the banning of performance enhancing steroids like testosterone and growth hormone because they confer an unnatural advantage to some athletes.
Synthetic anabolic-androgenic steroids (AAS) like testosterone are easily purchased online from overseas retailers. AAS are used by male and female athletes to increase muscle mass and strength. Frequent abuse can lead to several toxic side effects including kidney and heart disease. 25
Growth hormone is also abused as a way to increase aerobic performance and muscle mass. In the 1980s, athletes used cadaver pituitary growth hormone in order to increase the size of their muscles. This practice was reduced after increased worry about the development of Jacob-Creutzfeldt disease and with the increased availability of synthetic growth hormone produced in China26.

Comments: